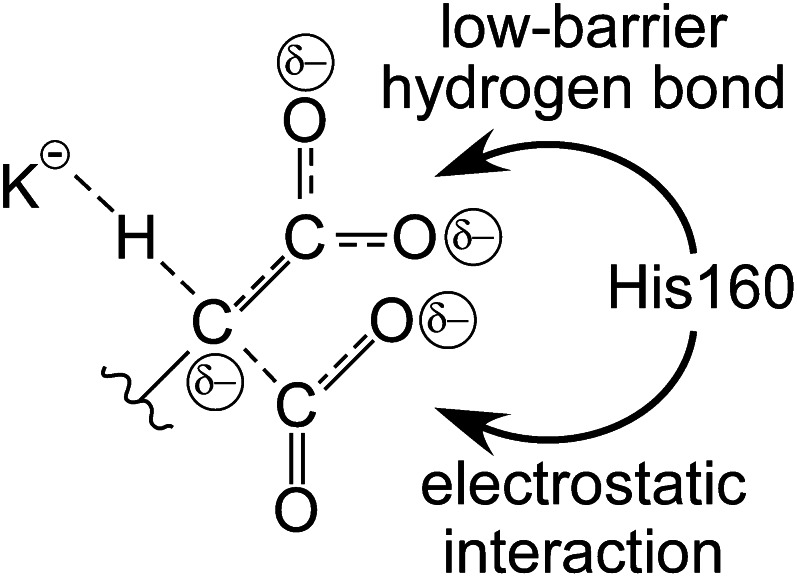
Figure 9.
Concerted deprotonation and carboxylation catalyzes the reaction by dispersing negative charge. As CO2 interaction is simultaneous with deprotonation, some of the negative charge developing on the γ carbon initiates bond formation with CO2, which in turn results in the appearance of negative charge on the CO2 oxygens. Additionally, the original glutamate carboxyl group can accept some negative charge from the γ carbon by formation of an aci-carboxylate structure. His160 could interact with either the aci-carboxylate moiety via a low-barrier hydrogen bond or with the negative charge on the incoming CO2 via an electrostatic interaction to effect catalysis.