Abstract
Women who have delivered an infant between 16 and 36 weeks’ gestation have an increased risk of preterm birth in subsequent pregnancies. The risk increases with more than 1 preterm birth and is inversely proportional to the gestational age of the previous preterm birth. African American women have rates of recurrent preterm birth that are nearly twice that of women of other backgrounds. An approximate risk of recurrent preterm birth can be estimated by a comprehensive reproductive history, with emphasis on maternal race, the number and gestational age of prior births, and the sequence of events preceding the index preterm birth. Interventions including smoking cessation, eradication of asymptomatic bacteriuria, progestational agents, and cervical cerclage can reduce the risk of recurrent preterm birth when employed appropriately.
Keywords: cerclage, prevention, progesterone, recurrent, preterm birth, risk estimation
Efforts to reduce the incidence of preterm birth cannot yet be called successful but have produced sufficient information to justify management suggestions for clinicians, not only for strategies that do not work but also for those that do. The quality and number of clinical trials of interventions intended to reduce the incidence and/or morbidity of preterm birth has risen substantially in recent years, yielding data that can improve care for women at risk.
An important advance has been the revision of the traditional model of preterm birth in which painful contractions or preterm membrane rupture were understood as the most common initial steps preceding cervical change. This concept has been challenged by observations from several clinical studies:
Drugs that arrest or inhibit uterine contractions can delay delivery but do not reliably reduce or prevent preterm birth.1–5
Assessment of contraction frequency is not a useful test to predict preterm birth,6 and its use to detect and arrest initial or recurrent episodes of preterm labor does not affect the rate of preterm birth.7–9
Antibiotics that eradicate microorganisms associated with preterm birth also do not reliably reduce, and may sometimes increase, the incidence of preterm birth, especially in women with intact membranes.10–17
Progestational agents such as 17α-hydroxy-progesterone caproate reduce recurrent preterm birth in some women with a prior preterm birth,18,19 especially those with early preterm birth20 and short cervix,21 but have no effect on preterm birth in multiple gestations.22,23
Cervical cerclage can reduce recurrent preterm birth in women with a prior preterm birth and short cervix24,25 but increases the risk of preterm birth in women with multiple gestation who also have a short cervix.24
These findings suggest the following 4 substantive changes in the “contractions change the cervix” construct of preterm birth:
Contractions are not the primary initial driver of most preterm births. Cervical ripening and decidual activation are more common first steps in preterm parturition.
Progesterone affects the steps leading to preterm birth in women who deliver very preterm (<32 weeks), a group characterized by inflammation-driven preterm birth and short cervix without apparent contractions. The antiinflammatory effect of progestins is therefore a likely mechanism of their effect on preterm birth.
Cerclage helps some of this same group,25 especially those with a short cervical length, suggesting cerclage may help more by preventing membrane prolapse and bacterial invasion of the amniotic cavity from vaginal flora than by making the cervix stronger.
The parturitional process clearly begins in many women before 20 weeks’ gestation, making this historical boundary between miscarriage and birth obsolete.26
These observations are the basis for an alternate model of preterm birth in which cervical ripening (short cervix), driven primarily by inflammation beginning in the early second trimester or even earlier, is the most common initial clinical manifestation of preterm parturition, especially in women with a previous preterm birth. Because preterm birth occurs in only 35–40% of all women with untreated short cervix27 and in 25–30% of women with a positive fetal fibronectin in the second trimester,28 many women apparently experience the initial phases of preterm parturition but do not progress to preterm birth. This is the model that underlies the recommendations discussed in the following text.
The contribution of prior preterm birth to preterm birth in general
Approximately 13 million of the more than 130 million babies born annually worldwide are born preterm, a global incidence of nearly 10%.29 There are more than 500,000 births before 37 weeks’ gestation in the United States each year, a rate of 12.7% in 2007.30 Of these, about 15% occur in women with a prior preterm birth.31 Current effective interventions could potentially eliminate as many as 35–50% of recurrent preterm births (n ~ 37,000).
Identification of women with a prior preterm birth
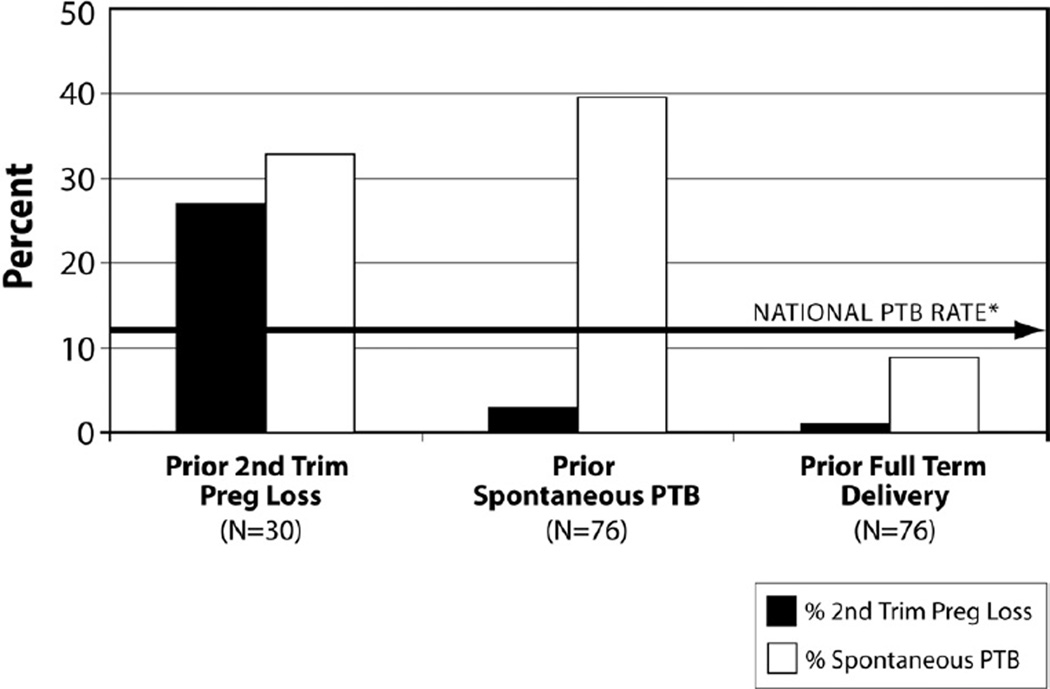
A thorough obstetrical history is essential to identify women with prior preterm birth. Unfortunately, the nomenclature used to describe pregnancy outcomes, expressed as gravidity, parity (births after 20 menstrual weeks’ gestation), and abortions (births before 20 weeks’), lacks a “clear epidemiologic, biologic, or clinical basis”26 and is inconsistent with recent obstetrical epidemiology: Women whose prior pregnancy ended between 16 and 20 weeks have a risk of recurrent preterm birth that equals or exceeds the recurrence risk for women whose prior preterm birth occurred after 20 weeks (Figure 1 from Edlow et al32). Women with a prior stillbirth are also often considered separately from those with a prior preterm birth, but their risk of subsequent spontaneous preterm birth is also increased.33 Thus, any woman with a prior birth between 160/7 and 366/7 weeks should be evaluated as possibly having had a preterm birth, whether the fetus was born alive or stillborn.
FIGURE 1. Pregnancy outcome after second trimester loss.
Subsequent pregnancy outcome in women with prior second trimester loss, prior spontaneous preterm birth, and prior birth at term.
Reproduced, with permission, from Edlow et al.32
A comprehensive reproductive history should record prior spontaneous abortions and elective terminations, including the gestational age and methods of termination. Contrary to common belief, population-based studies34–36 have found that elective pregnancy terminations in the first and second trimesters are associated with a very small but apparently real increase in the risk of subsequent spontaneous preterm birth (PTB).37 The mechanism is unknown. The gynecological history is also important. Cervical surgery (cold knife, laser conization, and loop-electrosurgical excision procedures [LEEP]) is also associated with increased risk of preterm birth.38
Because assisted reproduction techniques, including in vitro fertilization and all levels of ovulation promotion (clomiphene) and stimulation (gonado-tropins), have been associated with a 2-fold increased risk of PTB, the circumstances of conception of a prior pregnancy ending in preterm delivery should be reviewed.39,40 Superovulation followed by conception from fresh eggs in the same cycle has a greater risk of preterm birth than does use of frozen eggs.41
When the prior preterm birth occurred in a twin pregnancy, the risk of preterm birth in a subsequent singleton gestation varies according to the gestational age at delivery of the twins.42 The earlier the gestational age, the greater the risk of PTB in a subsequent singleton pregnancy, ranging from minimal if any increased risk for a twin birth after 34 weeks’ to as much as 40% when the prior twin birth occurred before 30 weeks.42,43
Pregnancies complicated by increased levels of maternal serum alpha-fetoprotein (MSAFP) unexplained by incorrect dates, multifetal gestation, or birth defects have an increased risk of adverse pregnancy outcomes, including preterm birth associated with abruption and preterm ruptured membranes.44 An elevated MSAFP may also occur with the demise of an unrecognized or vanishing twin, another risk factor for preterm birth.45
Document the clinical presentation of the prior preterm birth(s)
Preterm parturition is characterized by cervical ripening, decidual-membrane activation, and uterine contractions, any of which may predominate. The order in which these steps occurred can often be discerned from the history, offering clues to the etiology that can improve the prediction of recurrence risk. Obstetricians have traditionally been taught to ask about painful contractions as evidence that true labor has begun at term, but focusing on the presence and pain of contractions is inappropriate when taking a history of preterm parturition, in which cervical ripening and decidual-membrane activation are much more likely causes of the earliest signs and symptoms.
The most common sequence preceding preterm birth is cervical ripening (shortening of the cervix), followed by decidual-membrane activation and then contractions. This sequence is characterized by mild symptoms of pelvic pressure, premenstrual-like cramping, and increased vaginal discharge that occur over several days or weeks. The initial clinical examination reveals a soft, effaced cervix, with minimal dilation. Contractions are perceived as absent or mild because the cervix is already effaced and slightly dilated. Spotting or ruptured membranes may occur a few days later. The ultimate clinical presentation in these women may be pelvic pressure with advanced dilation, ruptured membranes, or contractions that seem inadequate to explain the advanced effacement and dilation. The risk of recurrent PTB for women who describe this sequence is high.
When preterm membrane rupture is the presenting complaint, a careful history may distinguish rupture that follows prolonged cervical ripening as described in the preceding text, from rupture after persistent spotting related to a vanishing twin or to a diagnostic amniocentesis, in which the recurrence risk is likely to be lower.
A history of painful contractions without any premenstrual symptoms suggests pressure against a closed, unripe cervix, as might occur when the stimulus for labor is acute (eg, an occult abruption). The recurrence risk in this setting will vary with the cause of abruption.
A confusing history (eg, of preterm birth in the first and third pregnancies with a birth at term in the second) raises the possibility of a uterine anomaly, in which preterm birth may be related to preterm separation of the placenta implanted on a uterine septum. We examine uterine anatomy with endovaginal ultrasound in these women. The recurrence risk will vary with the site of placental attachment.
Although preterm births are commonly labeled as being spontaneous or indicated, these categories often overlap, can be difficult to distinguish, and may be misleading when caring for a subsequent pregnancy. Review of the records of any prior preterm birth, regardless of the clinical circumstances, is therefore useful. This is important for births between 16 and 26 weeks, in which fetal anomalies, placental abnormalities, uterine anomalies, and spontaneous preterm parturition are often conflated.
Pathology reports should be reviewed for evidence of abruption, funisitis, and chorioamnionitis. Inflammation of the fetal membranes is found on the pathology report for most early preterm births, regardless of the process that led to preterm birth. When no inflammation is noted on pathological examination, we interpret that to suggest a relatively brief exposure of membranes to the vaginal flora, as might occur in cervical insufficiency.
Recurrent vs nonrecurrent preterm birth
Women with recurrent preterm birth are more likely to have a low prepregnancy weight (below 100 pounds, with a body mass index < 19.8 kg/m2) and to be African American than women with a single prior preterm birth.46–51 The risk of recurrent preterm birth increases as the gestational age of the previous preterm birth declines and as the number of previous preterm births increases, and thus is highest in women with more than 1 early preterm birth.52 Women with more than 1 preterm birth are also more likely to have prior early (<32 weeks) preterm birth, to demonstrate clinical and ultrasound evidence of short cervix (sonographic length <25 mm, and Bishop score >3), and to have a positive fibronectin at 22–24 weeks.53
A study of women with a prior preterm birth found that the likelihood of recurrent preterm delivery varied greatly, from less than 10% in women with a cervical length greater than 35 mm and a negative fibronectin at 22–24 weeks to more than 60% when the cervical length was less than 25 mm and the fibronectin test was positive at 22–24 weeks.54
Estimation of individual risk of recurrent preterm birth
The risk of recurrent preterm birth is commonly reported to be increased by 1.5- to 2-fold to 4-fold or more, depending on the population. The risk increases with the number of prior preterm births and as the gestational age decreases (Figure 2 from McManemy et al52).
FIGURE 2. Risk of recurrent preterm birth.
Risk of recurrent preterm birth in 19,025 women with 2 prior preterm births according to the order and gestational age of the previous preterm births.
Reproduced, with permission, from McManemy et al.52
There are 3 historical factors that have a substantial influence on the likelihood of recurrent preterm birth. All are immediately available without cost: maternal race (African American vs all others), the gestational age of the index preterm birth, and the number of previous preterm births. Each confers a 1.5- to 2-fold increase in the risk of recurrence, over and above the 1.5- to 2-fold increased risk in women with any prior preterm birth. Comparison of conservatively estimated personal recurrence risks for 2 hypothetical women, each with 2 prior preterm births, illustrates this approach as used in our clinics:
Patient A is a non-Hispanic white (NHW) woman whose first and second preterm births were both at 34 weeks. Her baseline risk is approximately 11% (overall rate of preterm birth for NHW women) and increases by approximately 1.5 for each prior PTB: 11% × 1.5 for the first PTB = 17.25% × 1.5 for the second PTB = an estimated risk of 26% in her third pregnancy.
Patient B is a non-Hispanic black (NHB) woman with 2 prior preterm births at 28 and 26 weeks’ gestation. Her baseline risk is 17% (the rate for NHB women in the United States) and increases by 1.5-fold because of the first preterm birth and then by another 1.5-fold because the birth was early, before 32 weeks. Her risk entering her second pregnancy was therefore approximately 35% (17% × 1.5 × 1.5). After a second PTB before 32 weeks, this same calculation yields a risk for her third pregnancy of more than 75% (35% × 1.5 × 1.5 = 79%).
These estimated risks are just approximations generated from published population risks and do not account for the sequence of term vs preterm births, in which the gestational age of the most recent birth predominates.49,52 More accurate estimates could be obtained from logistic regression analyses, but our simplistic method generates rate estimates that are consistent with reports in the literature.19,46,49,55,56 More specific information has been reported from studies of various tests for PTB risk,54,57–61 most usefully by Berghella et al59 (Table). Antenatal care decisions, such as the proper time to give antenatal corticosteroids, might be informed by knowledge of the risk of recurrent PTB according to the length of the cervix at various gestational ages (Table).
TABLE.
Predicted probability of delivery before week 32 by cervical length (millimeters) and gestational age in weeks at time of measurement
| Cervical length, mm |
Week of pregnancy | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | |
| 0 | 76.3 | 73.7 | 70.9 | 67.9 | 64.7 | 61.4 | 58.0 | 54.5 | 51.0 | 47.5 | 44.0 | 40.5 | 37.2 | 33.9 |
| 5 | 67.9 | 64.8 | 61.5 | 58.1 | 54.6 | 51.1 | 47.6 | 44.0 | 40.6 | 37.2 | 34.0 | 30.9 | 28.0 | 25.2 |
| 10 | 58.1 | 54.7 | 51.2 | 47.6 | 44.1 | 40.7 | 37.3 | 34.1 | 31.0 | 28.0 | 25.3 | 22.7 | 20.3 | 18.1 |
| 15 | 47.7 | 44.2 | 40.7 | 37.4 | 34.1 | 31.0 | 28.1 | 25.3 | 22.7 | 20.4 | 18.2 | 16.2 | 14.3 | 12.7 |
| 20 | 37.4 | 34.2 | 31.1 | 28.1 | 25.4 | 22.8 | 20.4 | 18.2 | 16.2 | 14.4 | 12.7 | 11.2 | 9.9 | 8.7 |
| 25 | 28.2 | 25.4 | 22.8 | 20.4 | 18.2 | 16.2 | 14.4 | 12.7 | 11.3 | 9.9 | 8.7 | 7.7 | 6.7 | 5.9 |
| 30 | 20.5 | 18.3 | 16.3 | 14.4 | 12.8 | 11.3 | 9.9 | 8.7 | 7.7 | 6.7 | 5.9 | 5.2 | 4.5 | 3.9 |
| 35 | 14.5 | 12.8 | 11.3 | 10.0 | 8.8 | 7.7 | 6.8 | 5.9 | 5.2 | 4.5 | 4.0 | 3.5 | 3.0 | 2.6 |
| 40 | 10.0 | 8.8 | 7.7 | 6.8 | 5.9 | 5.2 | 4.5 | 4.0 | 3.5 | 3.0 | 2.6 | 2.3 | 2.0 | 1.7 |
| 45 | 6.8 | 5.9 | 5.2 | 4.5 | 3.9 | 3.4 | 3.0 | 2.6 | 2.3 | 2.0 | 1.7 | 1.5 | 1.3 | 1.1 |
| 50 | 4.6 | 4.0 | 3.5 | 3.0 | 2.6 | 2.3 | 2.0 | 1.7 | 1.5 | 1.3 | 1.2 | 1.0 | 0.9 | 0.8 |
| 55 | 3.0 | 2.7 | 2.3 | 2.0 | 1.8 | 1.5 | 1.3 | 1.2 | 1.0 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 |
| 60 | 2.0 | 1.8 | 1.5 | 1.3 | 1.2 | 1.0 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | 0.4 | 0.3 |
Reproduced, with permission, from Berghella et al.59
Interventions to reduce the risk of recurrent preterm birth
Numerous strategies and treatments have been proposed to reduce the risk of recurrent preterm birth, but few have been found effective when tested in clinical trials. The interventions in the following text are grouped according to the strength of evidence supporting their use:
Interventions supported by firm evidence
Smoking-cessation programs: pregnant women are uniquely receptive to smoking-cessation programs, especially when physicians participate directly and repeatedly. Smoking-cessation programs in pregnancy have been reported to reduce the rate of preterm birth by 16% (relative risk, 0.84; 95% confidence interval [CI], 0.72–0.9862) to 31% (adjusted odds ratio [aOR], 0.69; 95% CI, 0.65–0.74).63
Screening and treatment for asymptomatic bacteriuria: screening for asymptomatic bacteriuria reduces recurrent urinary tract infections and may also reduce the risk of recurrent preterm birth.64
- Prophylactic administration of progestational agents: there is now sufficient evidence from at least 6 trials to support the prophylactic use of 17 alpha-hydroxyprogesterone caproate (17P), 250 mg given intramuscularly, weekly between 16 and 36 weeks, to women with prior spontaneous preterm birth between 20 and 366/7 weeks.19,65,66 This therapy reduces the risk of recurrent preterm birth by approximately 35% and is especially effective for women with a prior early preterm birth.19,20 17P does not reduce preterm birth risk in women with twin or triplet pregnancies.22,23 Progesterone supplementation appears to be safe for mothers and infants.65,67 There are many questions about clinical use of progestational agents to reduce recurrent preterm birth for which the literature is incomplete (eg, the selection of the appropriate candidates for treatment and the optimal pharmacological preparation). Current trials may answer some of these clinical dilemmas. Common questions are listed in the following text followed by our comments:
-
○Can progesterone supplementation be provided with oral progestational compounds? No. There is 1 randomized controlled trial in 150 women with prior preterm birth.68 This trial suggested efficacy, but larger studies are needed.
-
○Should vaginal progestational compounds be used to prevent preterm birth? Not at this time. Although some studies indicate a reduction in recurrent preterm birth in women treated with vaginal progestins,18,21 the largest trial did not show benefit.69 Until more definitive evidence is available, we prefer injectable 17P because a larger number of studied subjects support its efficacy65,66 and safety,67 because the benefit was clear, and, because 17P is given as an injection, there is no question about whether the study subjects actually received the medication.
-
○Should 17 P be offered to women with prior early (16–20 weeks) spontaneous birth? Because the clinical presentation of cervical insufficiency is indistinguishable from recurrent spontaneous preterm birth, 17P prophylaxis may be offered to all women with prior early (16–20 weeks) spontaneous preterm birth (Figure 3). The woman should understand that there are few data for this population.
-
○Should 17P be used in women after arrested preterm labor? No. Because only 1 small study has reported outcomes in women with arrested preterm labor treated with 17P, use in these women is not currently recommended.70
-
○Should 17P be used in women with a prior preterm birth followed by a term birth without any type of treatment who is now presenting in a third (or later) pregnancy? This question has not been directly addressed in the literature. The indication for 17P in this instance is an obstetrical history of spontaneous preterm birth, but the risk declines substantially if there is an intervening term birth. Current American College of Obstetricians and Gynecologists (ACOG) recommendations would support offering this woman 17P prophylaxis. Our recommendation would include consideration of the gestational age of the index preterm birth and the course of the subsequent pregnancy.52 Some examples include the following:
-
■First birth at 30 weeks with advanced cervical dilation at presentation, followed by a second pregnancy also complicated by early effacement and dilation, managed with reduced activity, tocolysis, and/or steroids, etc with delivery at term. We recommend 17P for this patient.
-
■First birth at 34 weeks after presenting with preterm ruptured membranes 1 week earlier, after a pregnancy complicated by intermittent light bleeding in the second trimester. The second pregnancy resulted in a birth at term without complications. The estimated recurrence risk in this patient may be as low as 13%.52 We would offer 17 P and recommend cervical sonography at 20–24 weeks.
-
■There are no current data on which to condition a recommendation about use of 17P on evidence of short or declining cervical length measurements. We do not use this strategy in our clinics, but it could become an appropriate option if supported by future adequately powered studies.
-
■
-
○Should progesterone supplementation be offered to women with a prior preterm birth related to obstetrical or medical complications such as fetal growth restriction, preeclampsia, or chronic hypertension? No. The literature supporting supplemental progesterone is entirely based on studies of women with a prior spontaneous preterm birth.
-
○Should progesterone supplementation be used in women with a twin pregnancy preceded by a spontaneous singleton preterm birth? This question has not been directly addressed in the literature. The indication for 17P in this instance is an obstetrical history of spontaneous preterm birth, but 17P is not effective in women with multifetal pregnancy.22,23 We recommend that this decision be individualized after a detailed history of the prior preterm birth.
-
○Should progesterone supplementation be used in women with a prior spontaneous preterm birth of a twin pregnancy? There are no clinical trial data to guide this decision in women with this combination of historical and current risk factors. Retrospective reports42,43 suggest that a history of spontaneous preterm birth of twins before 30–32 weeks without complicating diagnoses such as hypertension or fetal growth restriction confers an increased risk for preterm birth in subsequent singleton pregnancies. This decision should also be individualized after reviewing the gestational age and sequence of events in the twin gestation delivered preterm. A history of preterm birth in a triplet pregnancy does not constitute an indication for progesterone prophylaxis.
-
○
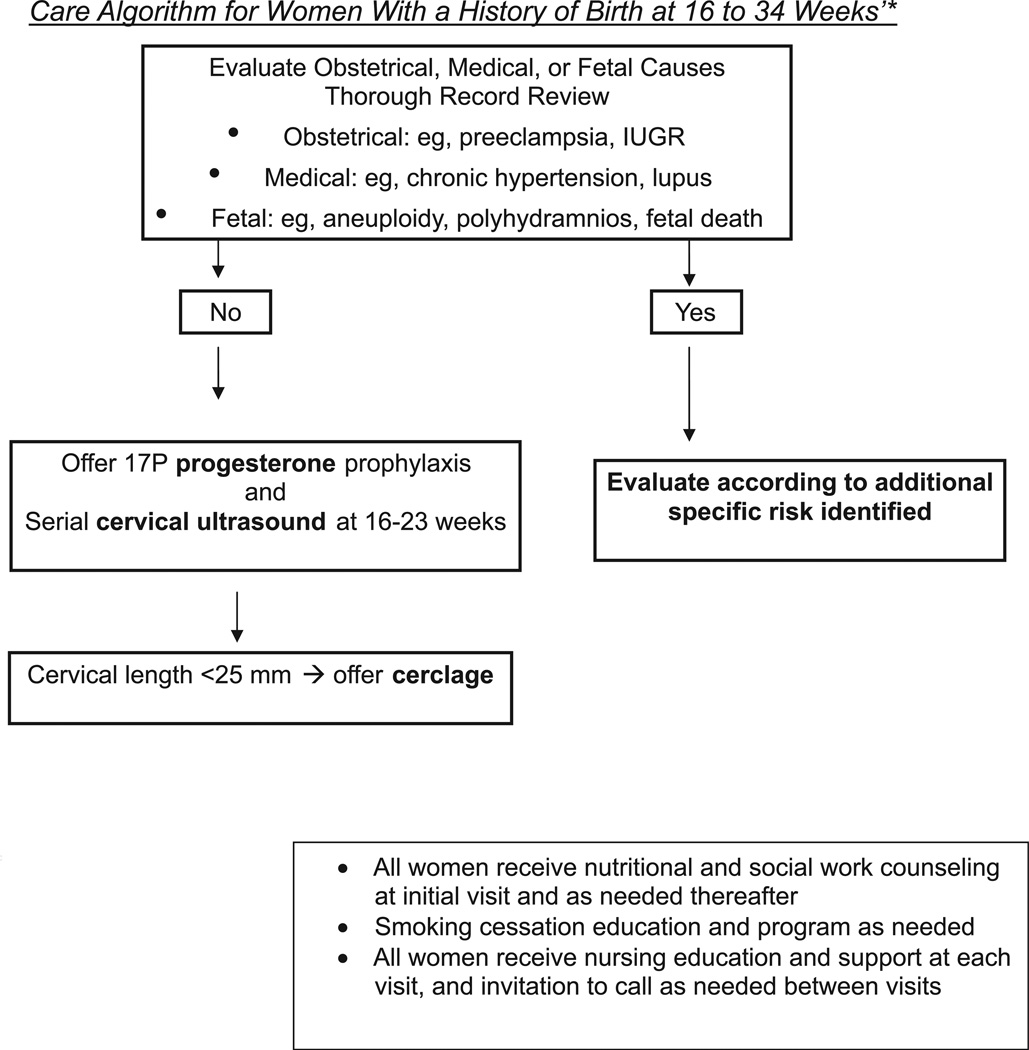
Cervical cerclage: a reduced risk of recurrent preterm birth for women with a short cervix treated in the current pregnancy with ultrasound-indicated cerclage has been reported.24 This conclusion was recently supported by a randomized clinical trial of 302 women with a prior preterm birth between 16 and 34 weeks (The Vaginal Ultrasound Cerclage Trial25). Recurrent preterm birth before 35 weeks occurred in 32% of women with cervical length less than 25 mm assigned to the cerclage group, compared with 42% of those assigned to the no-cerclage arm (OR, 0.67; 95% CI, 0.42–1.07; P = .053). The incidence of preterm birth less than 35 weeks’ was particularly decreased in the 64 women treated with cerclage whose cervical length less than 15 mm at randomization. Women with cervical lengths between 15 and 25 mm treated with cerclage delivered infants who experienced significantly less morbidity. The care algorithm in Figure 3 shows how we have integrated the results of the Vaginal Ultrasound Cerclage Trial into practice. We recommend 17P prophylaxis to all with a history of apparent spontaneous preterm birth and offer cerclage if the cervical length falls below 25 mm before 23 weeks’ gestation. We strongly encourage cerclage for women with prior preterm birth whose cervical length approaches 15 mm despite 17P prophylaxis. Of the 1014 women with a prior preterm birth between 16 and 34 weeks who were enrolled and followed up with cervical sonography in the Vaginal Ultrasound Cerclage Trial, 672 maintained a cervical length above 25 mm through 23 weeks’ gestation; only 16% of these women delivered <35 weeks.71 Results from randomized trials show similar rates of preterm birth in women with prior preterm births who undergo a history-indicated, prophylactic cerclage compared with those followed by ultrasound who were treated with cerclage only if the cervix shortened.72–74 Thus, cervical surveillance with sonography can select appropriate candidates who could benefit from cerclage while reducing needless surgical intervention and its complications (Figures 3 and 4).
Antenatal administration of corticosteroids: prenatal care for women with a prior preterm birth often requires a decision about whether and when antenatal corticosteroids should be given. Antenatal corticosteroids are indicated when the clinical scenario suggests that preterm birth is “imminent” (within 7–14 days) between 24 and 34 weeks’ gestation, but prediction of imminent preterm birth is difficult.75 We give a first course of betamethasone or dexamethasone to women with a prior preterm birth when there is current evidence of preterm parturition after 23 weeks (eg, spotting or bleeding), significant cervical change demonstrated by digital or ultrasound examination, persistent contractions, ruptured membranes, or a positive test for fetal fibronectin in a symptomatic woman. We consider in selected cases a second course of antenatal steroids in women with intact membranes who are judged to have a recurrent or continued threat of preterm delivery within the next 7–14 days and whose first course of betamethasone was given at least 14 days earlier and before 30 weeks’ gestation.76 Currently this is not recommended by ACOG. More research is needed to assess childhood outcomes.
FIGURE 3. Prenatal care algorithm for women with a prior preterm birth.
*see exceptions in Figure 4
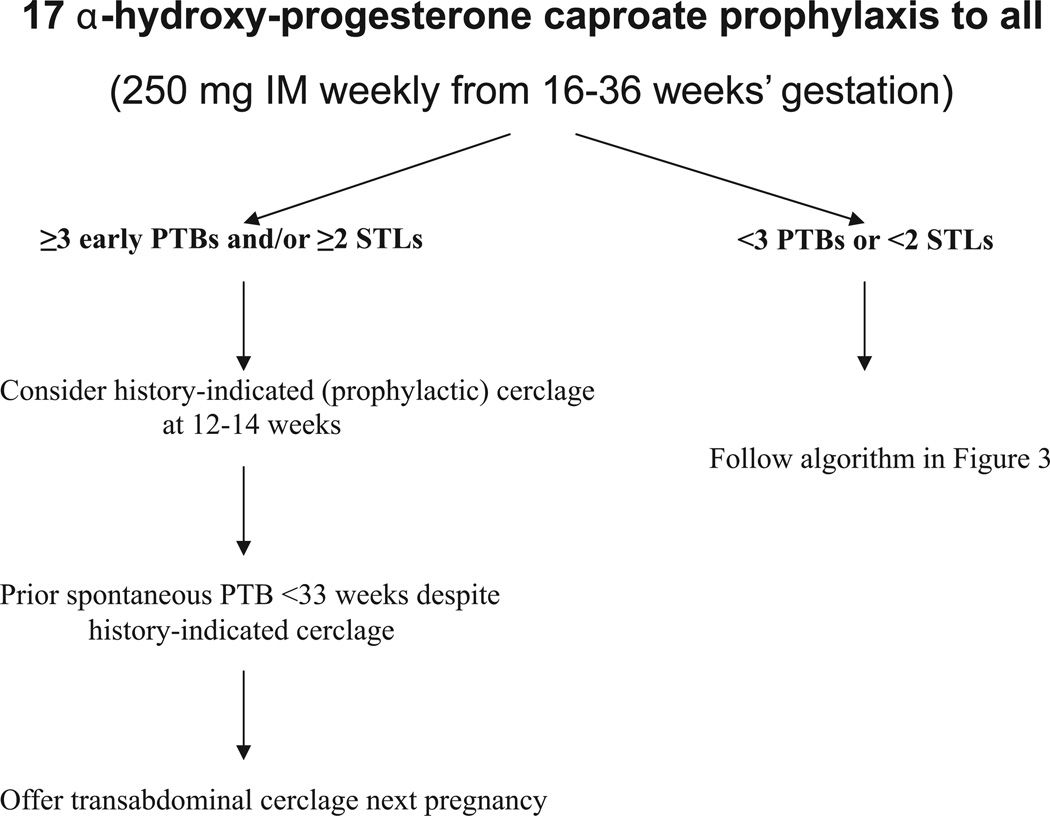
FIGURE 4. Care algorithm for asymptomatic women with multiple prior PTBs or STLs.
*see text
PTB, preterm birth; STL, second-trimester losses.
Interventions suggested to reduce the risk of recurrent preterm birth for which evidence is absent, minimal, or mixed
Surveillance of cervical length with transvaginal ultrasound has never been compared to digital or no cervical examination in a randomized trial of women with a prior preterm birth. Cervical ultrasound can reveal and quantify changes in the cervix sooner and more reproducibly than digital examination.77,78 However, the effect of cervical sonography on outcomes has not been demonstrated, and experience with ambulatory uterine contraction monitoring has shown that more information does not always improve outcomes. Women with a prior spontaneous preterm birth are followed up in our clinics with cervical length measurements beginning at 16 weeks. Examinations are repeated at approximately 2 week intervals through 24–26 weeks, with the expectation that preterm parturition will precede overt preterm labor or membrane rupture symptoms by 3–6 weeks. Examinations are done more frequently, or beyond 24 weeks, according to the clinical history of previous pregnancies, current symptoms, and the rate of cervical shortening in previous weeks.
Physical examination–indicated cerclage for dilated cervix and/or exposed membranes detected on manual physical exam at less than 24 weeks has been associated in a small randomized trial79 and a larger observational study80 with a reduced risk of preterm birth.
History-indicated cerclage at 12–14 weeks in women with 3 or more prior preterm births and/or second-trimester losses (STLs) may decrease recurrent preterm birth, according to a subanalysis of a large trial.81 It does not appear to be beneficial in women with fewer or later preterm births or STLs.82,83 Prior cerclage placement for an indication not supported by clinical trials is not an indication for placing a prophylactic cerclage in subsequent pregnancies.84 We follow up these women with transvaginal ultrasound cervical surveillance.
Transabdominal (TA) cerclage is performed for historical indications, usually because of the failure of 1 or more prophylactic vaginal cerclage procedures to prevent preterm birth in a woman with recurrent early preterm births. This therapy has not been evaluated in a prospective trial. Women who had a preterm birth less than 33 weeks despite a prior transvaginal cerclage had fewer recurrent preterm births after treatment with TA cerclage at 11–12 weeks in subsequent pregnancies.85 TA cerclage can now be safely performed laparoscopically in experienced hands, either before or during pregnancy at 10–12 weeks.
Maternal activity restriction is often recommended but there is insufficient evidence to support its use.86,87 It may cause harm by decreasing maternal muscle mass and increasing the risk of thromboembolism.
Maternal dietary fish consumption (omega-3 fatty acids [fish oil, Pikasol; Pronova Biocare, Aaslund, Norway]: 32% eicosapentaenoic acid [EPA], 23% docosahexaenoic acid [DHA], and 2 mg tocopherol per milliliter; 4 capsules/day: 1.3 g EPA and 0.9 g DHA, total 2.7 g/day; started ≥16 weeks, average 29–30 weeks) has been associated with prevention of preterm birth less than 37 weeks by 46% and preterm birth less than 34 weeks by 68% in women with a prior preterm birth less than 37 weeks and a singleton gestation in 1 trial,88 but a recent much larger randomized trial in women with prior preterm birth who also received 17P found no added benefit from omega-3 fatty acid supplements.89
Enhanced prenatal care and support, in the form of provider-initiated care and nurse-family partnerships has been suggested to reduce the risk of preterm birth, but the evidence is inconsistent and modest.90
Weekly manual cervical examinations in addition to education for women at high risk for preterm birth (≥10 on Creasy score) have not been associated with prevention of preterm birth.91–93
Supplemental calcium has not been studied specifically in women with a prior preterm delivery. Its use in other populations has not reduced preterm birth.94,95
Energy and protein supplementation may reduce the incidence of low birthweight (especially in malnourished women) but has no effect on preterm birth.96
Antibiotic treatment for women with a prior preterm birth and bacterial vaginosis has been suggested in secondary analyses to reduce the risk of recurrent preterm birth,97,98 but evidence is inconsistent.12–14,99,100 Antibiotic treatment of women with a prior preterm birth and a positive fetal fibronectin test10 and interconceptional antibiotics given to women with a prior preterm birth11 were both associated with an increased rate of recurrent preterm birth. Other trials in women at high risk for preterm birth have shown that antibiotics are ineffective at reducing recurrent preterm birth.14–16 We treat women with bacterial vaginosis who have symptoms but do not screen for it routinely.
Interventions that have been shown to be ineffective to reduce the risk of recurrent preterm birth
Nutritional supplements: randomized, placebo-controlled trials of vitamins C and E have not found any reduction in preterm birth.101,102
Early detection of preterm labor(PTL): small early trials suggested benefit, but subsequent randomized trials of programs to detect PTL, including provider-initiated care, frequent nurse contact, and outpatient home uterine contraction monitoring, did not reveal reduction of recurrent preterm birth.7
Contraction suppression: contraction suppression with various tocolytic drugs has been studied in women with prior preterm birth. This treatment reduces the frequency of contractions but has no effect on the rate of recurrent preterm birth or on neonatal outcomes.9,103–105 We do not use it as prophylaxis or after an episode of preterm labor. We believe the failure of this strategy to reduce the incidence of preterm birth in clinical trials and practice is not the result of inadequate dosage and/or ineffective drugs but instead provides evidence that uterine contractions are late manifestations of preterm parturition (see introductory text).
Periodontal care: periodontal care for pregnant women is apparently safe but did not affect the rate of preterm birth in 4 recent randomized trials.106–109
The context of prenatal care for women with a prior PTB
Women with a prior PTB may have lower rates of recurrent PTB when prenatal care includes specific attention to open communication between the patient and her caregivers. Lower rates of PTB have been reported in observational studies of provider-initiated contact,110 family nurse partnerships, and special programs for women with previous PTB.111 Because the clinical presentation of preterm parturition is more subtle than parturition at term, early signs and symptoms such as pelvic pressure and change in vaginal discharge may be easily dismissed unless patients and caregivers communicate easily.
The future of prenatal care for women with prior PTB
Selective use of progestational agents and cerclage112 is guided primarily by a clinical history of spontaneous PTB or repetitive midpregnancy loss, respectively. Neither is fully effective in all women with these historical indications. As experience with progesterone supplementation grows, prophylaxis maybe reserved for women with a prior PTB who also manifest another marker for PTB, such as a short cervix, a positive test for cervical inflammation, and/or who are found to have a genetic profile that suggests responsiveness to progesterone. Whether multiple interventions add more protection or can be selectively applied remains unclear. For example, 17P is currently recommended for a history of prior preterm birth, and many with this history will demonstrate short cervix. Is progesterone necessary in a woman with a prior preterm birth whose cervical length is normal? Are the beneficial effects of 17P for prior preterm birth and cerclage for a short cervix additive when they happen to be indicated for the same woman? Future use of effective interventions will follow discoveries in the pathophysiology of recurrent vs nonrecurrent preterm birth.
Key points that summarize the authors’ approach to prenatal care for women with a prior preterm birth
Obtain a thorough history and records for all prior pregnancies.
Try to determine the pathways that led to the prior PTB and categorize it as likely or unlikely to recur.
Estimate the woman’s personal risk of another PTB.
Identify and eliminate or minimize other risk factors.
Establish welcoming methods of communication between women with prior PTB and knowledgeable caregivers.
Assess personal and family resources and barriers to receiving care.
- Apply evidence-based interventions for women with prior spontaneous PTB such as the following:
-
○Smoking-cessation programs.
-
○Screen for and treat bacteriuria greater than 100,000 organisms per milliliter with appropriate antibiotics before conception, at the beginning of pregnancy, and later.
-
○17α-hydroxyprogesterone caproate 250 mg intramuscularly weekly from 16 weeks until 36 weeks.
-
○Cervical cerclage for women with short cervix (below 25 mm) or with visible membranes before 24 weeks.
-
○Corticosteroids (betamethasone 12 mg given intramuscularly and repeated in 24 hours) should be considered between 24 and 336/7 weeks for women with prior PTB who also manifest evidence of risk in the current pregnancy (eg, spotting or bleeding, rapid cervical effacement to cervical length <15 mm, PTL, visible membranes). For women in whom PTB does not occur within 3 weeks, risk remains very high (eg, new symptom or rupture of membranes) and are still less than 30 weeks, a single rescue course may be considered.
-
○
ACKNOWLEDGMENTS
Dr Berghella is currently participating in a prospective trial of vaginal progesterone to prevent preterm birth in high-risk women sponsored by Columbia Laboratories, makers of the product being studied in the trial. Neither of the authors receives money from, owns stock in, or speaks on behalf of Columbia Laboratories.
Contributor Information
Jay D. Iams, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, The Ohio State University Medical Center, Columbus, OH.
Vincenzo Berghella, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Thomas Jefferson University, Philadelphia, PA.
REFERENCES
- 1.Anotayanonth S, Subhedar NV, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev. 2006;2:CD004352. doi: 10.1002/14651858.CD004352.pub2. [DOI] [PubMed] [Google Scholar]
- 2.King JF, Flenady V, Papatsonis D, Dekker G, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database Syst Rev. 2002;3:CD002255. doi: 10.1002/14651858.CD002255. [DOI] [PubMed] [Google Scholar]
- 3.King JF, Flenady V, Cole S, Thornton S. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2005;2:CD001992. doi: 10.1002/14651858.CD001992.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2002;4:CD001060. doi: 10.1002/14651858.CD001060. [DOI] [PubMed] [Google Scholar]
- 5.Papatsonis D, Flenady V, Cole S, Liley H. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev. 2005;3:CD004452. doi: 10.1002/14651858.CD004452.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Iams JD, Newman RB, Thom EA, et al. Frequency of uterine contractions and the risk of spontaneous preterm delivery. N Engl J Med. 2002;346:250–255. doi: 10.1056/NEJMoa002868. [DOI] [PubMed] [Google Scholar]
- 7.Dyson DC, Danbe KH, Bamber JA, et al. Monitoring women at risk for preterm labor. N Engl J Med. 1998;338:15–19. doi: 10.1056/NEJM199801013380103. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists (ACOG) ACOG practice bulletin no. 31. Obstet Gynecol. 2001;98:709–716. [Google Scholar]
- 9.Sanchez-Ramos L, Huddleston JF. The therapeutic value of maintenance tocolysis: an overview of the evidence. Clin Perinatol. 2003;30:841–854. doi: 10.1016/s0095-5108(03)00104-0. [DOI] [PubMed] [Google Scholar]
- 10.Shennan A, Crawshaw S, Briley A, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fFN: the PREMET Study. BJOG. 2006;113:65–74. doi: 10.1111/j.1471-0528.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 11.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am J Obstet Gynecol. 2006;194:617–623. doi: 10.1016/j.ajog.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen GM, Bruinse HW. Prophylactic administration of clindamycin 2% vaginal cream to reduce the incidence of spontaneous preterm birth in women with an increased recurrence risk: a randomized placebo-controlled double-blind trial. BJOG. 1999;106:652–657. doi: 10.1111/j.1471-0528.1999.tb08363.x. [DOI] [PubMed] [Google Scholar]
- 13.Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487–493. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 14.Riggs MA, Klebanoff MA. Treatment of vaginal infections to prevent preterm birth: a metaanalysis. Clin Obstet Gynecol. 2004;47:796–807. doi: 10.1097/01.grf.0000141450.61310.81. [DOI] [PubMed] [Google Scholar]
- 15.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P. Prophylactic antibiotic administration in pregnancy to prevent infectious morbidity and mortality. Cochrane Database Syst Rev. 2002;4:CD002250. doi: 10.1002/14651858.CD002250. [DOI] [PubMed] [Google Scholar]
- 16.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007;1:CD000262. doi: 10.1002/14651858.CD000262.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews WW, Sibai BM, Thom EA, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin-positive women. Obstet Gynecol. 2003;101:847–855. doi: 10.1016/s0029-7844(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 18.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous PTB in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 19.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17-alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 20.Spong CY, Meis PJ, Thom EA, et al. Progesterone for prevention of recurrent preterm birth: impact of gestational age at previous delivery. Am J Obstet Gynecol. 2005;193:1127–1131. doi: 10.1016/j.ajog.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 22.Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 23.Caritis SN, Rouse DJ, Peaceman AM, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113:285–292. doi: 10.1097/AOG.0b013e318193c677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181–189. doi: 10.1097/01.AOG.0000168435.17200.53. [DOI] [PubMed] [Google Scholar]
- 25.Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for prevention of preterm birth in high-risk women with shortened mid-trimester cervical length. Am J Obstet Gynecol. 2009;201:375, e1–e8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creinin M, Simhan HN. Can we communicate gravidity and parity better? Obstet Gynecol. 2009;113:709–711. doi: 10.1097/AOG.0b013e3181988f8f. [DOI] [PubMed] [Google Scholar]
- 27.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–573. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 28.Goldenberg RI, Thom E, Moawad AH, et al. The preterm prediction study: fetal fibronectin, bacterial vaginosis and peripartum infection. Obstet Gynecol. 1996;87:656–660. doi: 10.1016/0029-7844(96)00034-8. [DOI] [PubMed] [Google Scholar]
- 29.Harris-Requejo J, Merialdi M. The global impact of preterm birth. In: Berghella V, editor. Preterm birth: prevention and management. Oxford, UK: Wiley-Blackwell; 2010. pp. 1–7. [Google Scholar]
- 30.Hamilton BE, Martin JA, Ventura SJ. National vital statistics reports. Web release. no 12. vol 57. Hyattsville, MD: National Center for Health Statistics; 2007. Births: preliminary data for 2007. Released March 18, 2009. [Google Scholar]
- 31.Petrini JR, Callaghan WM, Klebanoff M. Estimated effect of 17 alpha hydroxy-progesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. doi: 10.1097/01.AOG.0000150560.24297.4f. [DOI] [PubMed] [Google Scholar]
- 32.Edlow AG, Srinivas SK, Elovitz MA. Second trimester loss and subsequent pregnancy outcomes: what is the real risk? Am J Obstet Gynecol. 2007;197:581, e1–e6. doi: 10.1016/j.ajog.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Getahun D, Lawrence JM, Fasset JA, et al. The association between stillbirth in the first pregnancy and subsequent adverse perinatal outcomes. Am J Obstet Gynecol. 2009;201:378, e1–e6. doi: 10.1016/j.ajog.2009.06.071. [DOI] [PubMed] [Google Scholar]
- 34.Henriet L, Kaminski M. Impact of induced abortions on subsequent pregnancy outcome: the 1995 French national perinatal survey. BJOG. 2001;108:1036–1042. doi: 10.1111/j.1471-0528.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- 35.Ancel PY, Lelong N, Papiernik E, et al. for EUROPOP. History of induced abortion as a risk factor for PTB in European countries: results of the EUROPOP survey. Hum Reprod. 2004;19:734–740. doi: 10.1093/humrep/deh107. [DOI] [PubMed] [Google Scholar]
- 36.Moreau C, Kaminski M, Ancel PY, et al. for the EPIPAGE Group. Previous induced abortions and the risk of very preterm delivery: results of the EPIPAGE study. BJOG. 2005;112:430–437. doi: 10.1111/j.1471-0528.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 37.Shah PS, Zao J. Knowledge Synthesis Group of Determinants of Preterm/LBW Births. Induced termination of pregnancy and low birth weight and preterm birth: a systematic review and meta-analysis. BJOG. 2009;116:1425–1442. doi: 10.1111/j.1471-0528.2009.02278.x. [DOI] [PubMed] [Google Scholar]
- 38.Jakobsson M, Gissler M, Sainio S, et al. Preterm delivery after surgical treatment for cervical intraepithelial neoplasia. Obstet Gynecol. 2007;109:309–313. doi: 10.1097/01.AOG.0000253239.87040.23. [DOI] [PubMed] [Google Scholar]
- 39.Van Voorhis BJ. Outcomes from assisted reproductive technology. Obstet Gynecol. 2006;107:183–200. doi: 10.1097/01.AOG.0000194207.06554.5b. [DOI] [PubMed] [Google Scholar]
- 40.Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2007;109:967–977. doi: 10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- 41.Shih WG, Rushford DD, Bourne H, et al. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644–1653. doi: 10.1093/humrep/den150. [DOI] [PubMed] [Google Scholar]
- 42.Menard MK, Newman RB, Keenan A, Ebeling M. Prognostic significance of prior preterm twin delivery on subsequent singleton pregnancy. Am J Obstet Gynecol. 1996;174:1429–1432. doi: 10.1016/s0002-9378(96)70584-7. [DOI] [PubMed] [Google Scholar]
- 43.Facco FL, Nash K, Grobman WA. Are women who have had a preterm twin delivery at greater risk of preterm birth in a subsequent singleton pregnancy? Am J Obstet Gynecol. 2007;197:253, e1–e3. doi: 10.1016/j.ajog.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 44.Chandra S, Scott H, Dodds L, et al. Unexplained elevated maternal serum alpha-fetoprotein and/or human chorionic gonaodtropin and the risk of adverse outcomes. Am J Obstet Gynecol. 2003;189:775–781. doi: 10.1067/s0002-9378(03)00769-5. [DOI] [PubMed] [Google Scholar]
- 45.Shebl O, Ebner T, Sommergruber M, et al. Birth weight is lower for survivors of the vanishing twin syndrome: a case-control study. Fertil Steril. 2008;90:310–314. doi: 10.1016/j.fertnstert.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 46.Adams MM, Elam-Evans LD, Wilson HG, et al. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283:1591–1596. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 47.Esplin MS, O’Brien E, Fraser A, et al. Estimating recurrence risk of spontaneous preterm delivery. Obstet Gynecol. 2008;112:516–523. doi: 10.1097/AOG.0b013e318184181a. [DOI] [PubMed] [Google Scholar]
- 48.Kistka ZAF, Palomar L, Lee KA, et al. Racial disparity in the frequency of recurrence of PTB. Am J Obstet Gynecol. 2007;196:131, e1–e6. doi: 10.1016/j.ajog.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 49.Mercer BM, Goldenberg RL, Moawad A, et al. The preterm prediction study: effect of gestational age and cause of PTB on subsequent pregnancy outcome. Am J Obstet Gynecol. 1999;181:1216–1221. doi: 10.1016/s0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 50.Mercer B, Milluzzi B, Collin M. Periviable birth at 20–26 weeks of gestation: proximate causes, previous obstetric history and recurrence risk. Am J Obstet Gynecol. 2005;193:1175–1180. doi: 10.1016/j.ajog.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 51.Mercer BM, Macpherson CA, Goldenberg RL, et al. Are women with recurrent spontaneous PTBs different from those without such history? Am J Obstet Gynecol. 2006;194:1176–1185. doi: 10.1016/j.ajog.2006.01.069. [DOI] [PubMed] [Google Scholar]
- 52.McManemy J, Cooke E, Amon E, et al. Recurrence risk for preterm delivery. Am J Obstet Gynecol. 2007;196:576, e1–e6. doi: 10.1016/j.ajog.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 53.Goldenberg RL, Iams JD, Mercer BM, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous PTBs. NICHD MFMU Network. Am J Public Health. 1998;88:233–238. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: recurrence risk of spontaneous PTB. Am J Obstet Gynecol. 1998;178:1035–1040. doi: 10.1016/s0002-9378(98)70544-7. [DOI] [PubMed] [Google Scholar]
- 55.Kaltreider DF, Kohl S. Epidemiology of preterm delivery. Clin Obstet Gynecol. 1980;23:17–31. doi: 10.1097/00003081-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Carr-Hill, Hall MH. The repetition of spontaneous preterm labour. Br J Obstet Gynaecol. 1985;92:921–928. doi: 10.1111/j.1471-0528.1985.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 57.Kagan KO, To M, Tsoi E, Nicolaides KH. Preterm birth: the value of sonographic measurement of cervical length. BJOG. 2006;113(Suppl 3):52–526. doi: 10.1111/j.1471-0528.2006.01124.x. (Review). Erratum in: BJOG 2008;115:674-5. [DOI] [PubMed] [Google Scholar]
- 58.Celik E, To M, Gajewska K, Smith GCS, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized assessment. Ultrasound Obstet Gynecol. 2008;31:549–554. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 59.Berghella V, Roman A, Daskalakis C, et al. Gestational age at cervical length measurement and incidence of preterm birth. Obstet Gynecol. 2007;110:311–317. doi: 10.1097/01.AOG.0000270112.05025.1d. [DOI] [PubMed] [Google Scholar]
- 60.Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193:1170–1174. doi: 10.1016/j.ajog.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 61.Crane JMG, Hutchens D. Use of transvaginal ultrasonography to predict preterm birth in women with a history of PTB. Ultrasound Obstet Gynecol. 2008;32:640–645. doi: 10.1002/uog.6143. [DOI] [PubMed] [Google Scholar]
- 62.Lumley J, Chamberlain C, Dowswell T, et al. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2009;3:CD001055. doi: 10.1002/14651858.CD001055.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polakowski LL, Akinbami LJ, Mendola P. Prenatal smoking cessation and the risk of delivering preterm and small-for-gestational-age newborns. Obstet Gynecol. 2009;114:318–325. doi: 10.1097/AOG.0b013e3181ae9e9c. [DOI] [PubMed] [Google Scholar]
- 64.Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001;2:CD000490. doi: 10.1002/14651858.CD000490. [DOI] [PubMed] [Google Scholar]
- 65.Meis PJ. Society for Maternal-Fetal Medicine. 17 Hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol. 2005;105(5 Pt 1):1128–1135. doi: 10.1097/01.AOG.0000160432.95395.8f. [DOI] [PubMed] [Google Scholar]
- 66.Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing PTB. Cochrane Database Syst Rev. 2006;1:CD004947. doi: 10.1002/14651858.CD004947.pub2. [DOI] [PubMed] [Google Scholar]
- 67.Northen AT, Norman GS, Anderson K, et al. Follow-up of children exposed in utero to 17 alpha-hydroxyprogesterone caproate compared with placebo. Obstet Gynecol. 2007;110:865–872. doi: 10.1097/01.AOG.0000281348.51499.bc. [DOI] [PubMed] [Google Scholar]
- 68.Rai P, Rajaram S, Goel N, Gopalakrishnan RA, Agarwal R, Mehta S. Oral micronized progesterone for prevention of preterm birth. Internat J Gynecol Obstet. 2009;104:40–43. doi: 10.1016/j.ijgo.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 69.O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent PTB: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 70.Facchinetti F, Paganelli S, Comitini G, et al. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453, e1–e4. doi: 10.1016/j.ajog.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 71.Owen J, Szychowski J, Hankins G, et al. Does midtrimester cervical length ≥25 mm predict preterm birth in high-risk women? Am J Obstet Gynecol. 2010 doi: 10.1016/j.ajog.2010.06.025. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Althuisius SM, Dekker GA, van Geijn HP, Bekedam DJ, Hummel P. Cervical incompetence prevention randomized cerclage trial (CIPRACT): study design and preliminary results. Am J Obstet Gynecol. 2000;183:823–829. doi: 10.1067/mob.2000.108874. [DOI] [PubMed] [Google Scholar]
- 73.Simcox R, Seed PT, Bennett P, et al. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial) Am J Obstet Gynecol. 2009;200:623, e1–e6. doi: 10.1016/j.ajog.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Kassanos D, Salamalekis E, Vitoratos N, Panayotopoulos N, Loghis C, Creatsas C. The value of transvaginal ultrasonography in diagnosis and management of cervical incompetence. Clin Exp Obstet Gynecol. 2001;28:266–268. [PubMed] [Google Scholar]
- 75.Mercer B, Egerman R, Beazley D, et al. Antenatal corticosteroids in women at risk for preterm birth: a randomized trial. Am J Obstet Gynecol. 2001;184:S6. [Google Scholar]
- 76.Garite TJ, Kurtzman J, Maurel K, et al. Impact of a ‘rescue course’ of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol. 2009;200:248, e1–e9. doi: 10.1016/j.ajog.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 77.Gomez R, Galasso M, Romero R, et al. Ultrasonographic examination of the cervix is better than cervical digital examination as a predictor of preterm delivery in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1994;171:956. doi: 10.1016/0002-9378(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 78.Goldberg J, Newman RB, Rust PF. Interob-server reliability of digital and endovaginal ultrasonographic cervical length measurements. Am J Obstet Gynecol. 1997;177:853. doi: 10.1016/s0002-9378(97)70282-5. [DOI] [PubMed] [Google Scholar]
- 79.Althuisius SM, Dekker GA, Hummel P, van geijn HP. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2003;189:907–910. doi: 10.1067/s0002-9378(03)00718-x. [DOI] [PubMed] [Google Scholar]
- 80.Pereira L, Cotter A, Gómez R, et al. Expectant management compared with physical examination-indicated cerclage (EM-PEC) in selected women with a dilated cervix at 14(0/7)–25(6/7) weeks: results from the EM-PEC international cohort study. Am J Obstet Gynecol. 2007;197:483, e1–e8. doi: 10.1016/j.ajog.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 81.MCR/RCOG Working Party on Cervical Cerlage. Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomized trial of cervical cerclage. Br J Obstet Gynecol. 1993;100:516–523. doi: 10.1111/j.1471-0528.1993.tb15300.x. [DOI] [PubMed] [Google Scholar]
- 82.Rush RW, Issacs S, McPherson K, Jones L, Chalmers I, Grant A. A randomized controlled trial of cervical cerclage in women at high risk of preterm delivery. Br J Obstet Gynecol. 1984;91:724–730. doi: 10.1111/j.1471-0528.1984.tb04840.x. [DOI] [PubMed] [Google Scholar]
- 83.Lazar P, Gueguen S, Dreyfus J, Renaud R, Pontonnier G, Papiernik E. Multicentre controlled trial of cervical cerclage in women at moderate risk of preterm delivey. Br J Obstet Gynecol. 1984;91:731–735. doi: 10.1111/j.1471-0528.1984.tb04841.x. [DOI] [PubMed] [Google Scholar]
- 84.Pelham J, Lewis D, Berghella V. Prior cerclage: to repeat or not to repeat? Am J Perinat. 2008;25:417–420. doi: 10.1055/s-2008-1075037. [DOI] [PubMed] [Google Scholar]
- 85.Davis G, Berghella V, Talucci M, Wapner RJ. Patients with a prior failed transvaginal cerclage: a comparison of obstetric outcomes with either transabdominal or transvaginal cerclage. Am J Obstet Gynecol. 2000;183:836–839. doi: 10.1067/mob.2000.108837. [DOI] [PubMed] [Google Scholar]
- 86.Sosa C, Althabe F, Belizán J, Bergel E. Bed rest in singleton pregnancies for preventing PTB. Cochrane Database Syst Rev. 2004;1:CD003581. doi: 10.1002/14651858.CD003581.pub2. [DOI] [PubMed] [Google Scholar]
- 87.Hobel CJ, Ross MG, Bermis RL, et al. The West Los Angeles preterm birth prevention project. I. Program impact on high-risk women. Am J Obstet Gynecol. 1994;170:54–62. doi: 10.1016/s0002-9378(94)70384-1. [DOI] [PubMed] [Google Scholar]
- 88.Olsen SF, Secher NJ, Tabor A, Weber T, Walker JJ, Gluud C. Randomized clinical trials of fish oil supplementation in high risk pregnancies. Br J Obstet Gynecol. 2000;107:382–395. doi: 10.1111/j.1471-0528.2000.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 89.Harper M, Thom E, Klebanoff MA, et al. Omega-3 fatty acid supplementation to prevent recurrent preterm birth. Obstet Gynecol. 2010;115:234–242. doi: 10.1097/AOG.0b013e3181cbd60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hodnett ED, Fredericks S. Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database Syst Rev. 2003;3:CD000198. doi: 10.1002/14651858.CD000198. [DOI] [PubMed] [Google Scholar]
- 91.Mueller-Heubach E, Riddick D, Barnett B, Bente R. Preterm birth prevention: evaluation of a prospective controlled trial. Am J Obstet Gynecol. 1989;160:1172–1178. doi: 10.1016/0002-9378(89)90183-x. [DOI] [PubMed] [Google Scholar]
- 92.Main DM, Richardson DK, Hadley CB, Gabbe SG. Controlled trial of a preterm labor detection program: efficacy and costs. Obstet Gynecol. 1989;74:873–877. [PubMed] [Google Scholar]
- 93.Collaborative Group on Preterm Birth Prevention. Multicenter randomized controlled trial of a preterm birth prevention program. Am J Obstet Gynecol. 1993;169:352–366. doi: 10.1016/0002-9378(93)90087-y. [DOI] [PubMed] [Google Scholar]
- 94.Levine RJ, Hauth JC, Curet LB, et al. Trial of calcium to prevent preeclampsia. N Engl J Med. 1997;337:69–76. doi: 10.1056/NEJM199707103370201. [DOI] [PubMed] [Google Scholar]
- 95.Hofmeyer GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2006;3:CDC001059. doi: 10.1002/14651858.CD001059.pub2. [DOI] [PubMed] [Google Scholar]
- 96.Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev. 2003;4:CD000032. doi: 10.1002/14651858.CD000032. [DOI] [PubMed] [Google Scholar]
- 97.Hauth JC, Goldenberg RI, Andrews WW, et al. Reduced incidence of preterm delivery with metronidazole and erthromycin in women with bacterial vaginosis. N Engl J Med. 1995;333:1732–1736. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 98.McDonald HM, O’Loughlin JA, Vigneswaran R, et al. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis: flora (Garnerella vaginalis): a randomized, placebo controlled trial. Br J Obstet Gynaecol. 1997;104:1391–1397. doi: 10.1111/j.1471-0528.1997.tb11009.x. [DOI] [PubMed] [Google Scholar]
- 99.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 200;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 100.Gighangi PB, Ndinya-Achola JO, Ombete J, et al. Antimicrobial prophylaxis in pregnancy: a randomized, placebo-controlled trial with cefetamet-pivoxil in pregnant women with poor obstetrical history. Am J Obstet Gynecol. 1997;177:680–684. doi: 10.1016/s0002-9378(97)70164-9. [DOI] [PubMed] [Google Scholar]
- 101.Rumbold AR, Crowther CA, Haslam RR, et al. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 102.Poston L, Briley AL, Seed PT, et al. Vitamin C and vitamin E in pregnant women at risk for preeclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 103.Dodd JM, Crowther CA, Dare MR, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database Syst Rev. 2006;1:CD003927. doi: 10.1002/14651858.CD003927.pub2. [DOI] [PubMed] [Google Scholar]
- 104.Whitworth M, Quenby S. Prophylactic oral betamimetics for preventing preterm labour in singleton pregnancies. Cochrane Database Syst Rev. 2008;1:CD006395. doi: 10.1002/14651858.CD006395.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gaunekar NN, Crowther CA. Maintenance therapy with calcium channel blockers for preventing preterm birth after threatened preterm labour. Cochrane Database Syst Rev. 2004;3:CD004071. doi: 10.1002/14651858.CD004071.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Michalowicz BS, Hodges JS, Di Angelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 107.Offenbacher S, Beck J, Jared H, et al. Effects of periodontal therapy onrate of preterm delivery: a randomized-controlled trial. Obstet Gynecol. 2009;114:551–559. doi: 10.1097/AOG.0b013e3181b1341f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Macones GA, Parry S, Nelson DB, et al. Treatment of local periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS) Am J Obstet Gynecol. 2010;202:147, e1–e8. doi: 10.1016/j.ajog.2009.10.892. [DOI] [PubMed] [Google Scholar]
- 109.Newnham JP, Newnham IA, Ball CM, et al. Treatment of periodontal disease during pregnancy: a randomized controlled trial. Obstet Gynecol. 2009;114:1239–1248. doi: 10.1097/AOG.0b013e3181c15b40. [DOI] [PubMed] [Google Scholar]
- 110.Moore ML, Meis PJ, Ernest JM, et al. A randomized trial of nurse intervention to reduce preterm and low birth weight births. Obstet Gynecol. 1998;91:656–561. doi: 10.1016/s0029-7844(98)00012-x. [DOI] [PubMed] [Google Scholar]
- 111.Newman RB, Sullivan SA, Menard MK, et al. South Carolina Partners for Preterm Birth Prevention: a regional perinatal initiative for the reduction of premature birth in a Medicaid population. Am J Obstet Gynecol. 2008;199:393, e1–e8. doi: 10.1016/j.ajog.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 112.Berghella V. Novel developments on cervical length screening and progesterone for preventing preterm birth. BJOG. 2009;116:182–187. doi: 10.1111/j.1471-0528.2008.02008.x. [DOI] [PubMed] [Google Scholar]