Abstract
The capacity to imitate facial gestures is highly variable in rhesus macaques and this variability may be related to differences in specific neurobehavioral patterns of development. This study evaluated the differential neonatal imitative response of 41 macaques in relation to the development of sensory, motor, and cognitive skills throughout the 1st month of life. The results show that infants who imitate facial gestures display more developed skills in goal-directed movements (reaching–grasping and fine hand motor control) than non-imitators. These differences might reflect, at least in part, the differential maturation of motor chains in the parietal and motor cortices, which partly overlap with those of the mirror neuron system. Thus, neonatal imitation appears to be a predictor of future neurobehavioral development.
Thirty years ago, the first study to report the capacity of human infants to imitate facial gestures was published (Meltzoff & Moore, 1977). Two basic facial gestures have been consistently reported to be imitated by infants: mouth opening and tongue protrusion. These results have been replicated and partially confirmed by other investigations (Abravanel & Sigafoos, 1984; Legerstee, 1991; Meltzoff & Moore, 1992). They also revealed that imitative abilities are present from the first hour after birth and disappear after 2–3 months of age (Fontaine, 1984). Further experiments showed that the capacity of infants to imitate gestures might not be limited to tongue protrusion and mouth opening. Infants in fact are also able to imitate facial expressions (Field, Woodson, Greenberg, & Cohen, 1982) and hand gestures (Fontaine, 1984; but see also Anisfeld, 1996, for a critical evaluation). The specificity of the imitated responses indicates an innate capacity in infants to match the observed biological movements to the motor representation of their own bodies.
The discovery of neonatal imitation in other primates (Bard, 2005, 2007; Bard & Russell, 1999; Ferrari, Visalberghi, Fogassi, Ruggiero, & Suomi, 2006; Myowa-Yamakoshi, Tomonaga, Tanaka, & Matsuzawa, 2004) has represented a breakthrough in primate research with several implications for developmental psychology. Chimpanzees’ ability to imitate human facial gestures is, similar to humans, specific for tongue protrusion and mouth opening. Remarkably, the time period in which infant chimpanzee imitate is around 2 months, also similar to that reported for human infants (Myowa-Yamakoshi et al., 2004). A more recent study explored this phenomenon by testing five chimpanzees in the first 2 weeks of life (Bard, 2007), and the data confirmed previous observations. Moreover, Bard (2007) found that contextual features, for example, a communicative rather than a structured paradigm, appeared to influence the chimpanzees’ behavior. In particular, chimpanzee infants showed higher imitative responses to communicative gestures, including tongue clicks. More extensive data derive from a recent study on neonatal imitation in infant rhesus macaques (Ferrari et al., 2006). One- to 3-day-old macaque infants imitated lipsmacking, a typical macaque affiliative gesture, and tongue protrusion. In contrast to humans and chimpanzees, macaques display imitative abilities apparently for only a few days. Moreover, it has been argued that, considering the faster motor development of macaques compared to humans and chimpanzees, the temporal window in which this behavior is present in macaques is comparable with that reported for the other two species (Ferrari et al., 2006).
Understanding the developmental trajectory of this phenomenon might be useful from a comparative perspective, as it would provide important information on the basic mechanisms involved as well as on the possible functions. Even more important would be the comparison between humans and macaques as the latter species, having a shorter developmental trajectory, might be able to serve as a model for possible links between phenomena that appear at birth and future behavioral and cognitive processes. In fact, one of the advantages of investigating development in macaques is the possibility to observe, in a relatively short time period, changes in cognitive-motor skills and the impact of early experiences on such skills. This approach has been successfully applied in the past as a primate model of mother–infant interactions and to understand the effects of early mother deprivation on social-cognitive development of the infants (Suomi, 1999).
An interesting aspect of neonatal imitation is interindividual variability in infant responses. A critical review of the literature shows that about half of the tested human infants imitate mouth opening or tongue protrusion (Heimann, Nelson, & Schaller, 1989). On the other hand, some newborns do not imitate or do not complete the testing. Often, human infants that are difficult or distracted during testing are excluded from analysis. Similarly to humans, macaques also show substantial interindividual differences in neonatal responses to human facial gestures. However, it is not clear if these differences are specific to the stimuli and context in which infants are tested and therefore not linked to other cognitive processes or, alternatively, if the newborn response reflects a specific individual cognitive component of a broader pattern of development. In the first case, one might expect that interindividual differences would not predict any specific pattern of development. In the latter case, one might expect that newborn imitation is part of a more general pattern of development and thus might be a valid predictor of specific cognitive and behavioral achievements that take place later in life.
The present research will attempt to investigate the phenomenon of neonatal imitation within a broader perspective of development. Neonatal imitation in humans has been amply studied but the information about its connection with other aspects of development has been very limited. This lack of knowledge can be clearly attributed to the difficulty in following cognitive development longitudinally in the same subjects. Thus, our study on macaques may offer an important opportunity for gathering such information. The strategy adopted will be that of following single subjects at different stages of development.
We therefore investigated the differential imitative neonatal response of newborn macaques in the 1st week of life in relation to neurobehavioral responses assessed within the 1st month of life by a modified version of the Brazelton Neonatal Assessment Scales and the Bayley Scales of Infant Development (Schneider & Suomi, 1992). More specifically, we were interested in understanding whether imitative responses would be significantly correlated with the development of behaviors that are controlled by different neural circuits in cortical and subcortical structures (Herschkowitz, 2000; Rizzolatti & Luppino, 2001; Touwen, 1995).
In particular, our interest in the development of intentional actions such as reaching–grasping was based on the fact that the capacity to plan and organize actions represents an important landmark in infant development and is underpinned by specific parietal-premotor cortical areas (Jeannerod et al., 1995; Rizzolatti, Fadiga, Fogassi, & Gallese, 2002; Rizzolatti & Luppino, 2001; Von Hofsten, 2004). Second, reaching and grasping, in contrast to other reflex-like movements or gaze control, must rely on mechanisms that integrate action and perception for the perceptual guidance of the movements. This aspect is crucial in cognitive neuroscience as growing empirical evidence has demonstrated that cortical areas involved in the production of actions are also involved in the perceptions of others’ actions (Rizzolatti & Craighero, 2004; Rizzolatti, Fogassi, & Gallese, 2001). This link between action and perception is the core problem in imitative behaviors and is likely to be underpinned by a shared neural network (Iacoboni et al., 1999; Rizzolatti et al., 2001).
We hypothesize that neonatal imitation relies on a cortical mechanism that involves parietal and premotor cortical areas. As part of these areas are recruited for the production of reaching–grasping actions, we predict that group differences in neonatal behaviors are associated with differences in reaching–grasping but not with other behaviors such as palmar reflexes, visual attention, body posture and emotionality that are controlled by other cortical and subcortical brain structures.
Method
Subjects and Housing
Subjects were 41 infant rhesus macaques (Macaca mulatta), 25 males and 16 females. Some neonatal imitation data from 21 of these subjects have previously been published elsewhere (Ferrari et al., 2006). An unrelated experimental protocol required all subjects to be separated from their mothers on Day 1 postpartum. They were reared in a nursery facility according to procedures described by Ruppenthal, Arling, Harlow, Sackett, and Suomi (1976; see also Ferrari et al., 2006, for further details). Infants were individually housed in plastic incubators (51 × 38 × 43 cm) that each contained a 25-cm-high inanimate “surrogate mother.” During the 1st week of life, the surrogate mother was composed of a 16.5-cm circumference polypropylene cylinder, wrapped in fleece fabric and attached by a flexible metal component to an 11.5-cm-wide circular metal base. From the 2nd week onward, infants were provided with a hanging surrogate mother (see also Dettmer, Novak, Meyer, Ruggiero, & Suomi, in press) consisting of a plastic cylinder core (20 cm high and 19 cm circumference) with a wide soft cloth cover (20 × 25 cm).
Neonatal Imitation Tests
In the previous study, we demonstrated that the most effective gestures imitated by infants were tongue protrusion and lipsmacking (Ferrari et al., 2006). For this reason, we tested infants with only these two gestures to reduce the potential stress generated by a prolonged testing. A third, nonbiological stimulus condition was also run, but it is not discussed here. Subjects were tested at the ages of 1, 3, 5, and 7 days or, due to experimental constraints, 1 day after these days. Subjects from the previous study were not tested on Day 5. We collected data on 30 infants on Day 1, 31 on Day 3, 19 on Day 5, and 32 on Day 7. Infants were tested when awake and alert ca. 30 min after feeding. During testing, infants were wrapped in and often clung to pieces of fleece fabric. This arrangement visibly calmed the infants and minimized their distress.
Three experimenters were involved in the data collection. One experimenter held the infant monkey in his or her hands, the second (the demonstrator) served as the source of the stimuli, and the third videotaped the experiment and informed the demonstrator of the onset and offset of the different phases of each trial. Individual demonstrators were randomly assigned to conditions and infants but remained consistent within infants. A video camera (Sony Digital Video Camcorder ZR600, Lake Success, NY; positioned 0.5 m behind the demonstrator) recorded the monkeys’ behavior with only the monkey in view.
Each trial started with a 40-s baseline, in which the experimenter faced the infant with a passive/neutral expression. We then presented each stimulus for 20 s: tongue protrusion (TP; protrusion with maximal extension and retraction of the tongue, ca. 7 openings per 20 s) or lipsmacking (LPS; a high-frequency opening and closing of the mouth without sound production, ca. 100 openings per 20 s). Each presentation phase was followed by 20 s of passive/neutral expression. This stimulus still-face sequence was repeated three times, with the only exception that the final still-face period was 40 s long (total testing time per trial: 3 min). All conditions were randomly presented on each testing day with at least 2 hr between individual sessions.
Testing procedures for the 21 previously tested infants differed slightly from the procedures described earlier. In particular, these infants were only tested once a day, and each gesture was presented for only one 20-s period followed by a 20-s still-face period. For further details, see Ferrari et al. (2006).
Behavioral Analysis
All tapes were digitally analyzed by a coder not blind to the experimental condition using all occurrence sampling for all lipsmacking and tongue protrusion behaviors. We counted the frequency of lipsmacking behaviors (opening and closing of the mouth; see also Ferrari et al., 2006) and tongue protrusion behaviors (forward movements of the tongue that cross the inner edge of the lower lip; see also Ferrari et al., 2006) in each phase. Forty percent of sessions were also coded by a second coder, also not blind to the experimental condition, and achieving high interobserver reliability (Cohen’s kappa = .95). A further 10% of sessions were coded by a coder blind to the experimental condition showing high consistency between blind and non-blind codings (Cohen’s kappa = .86).
Infant Neurobehavioral Assessment
All infants were tested on a 20-min battery of developmental tests administered on Days 7, 14, 21, and 30 postpartum. The purpose of the original test battery (Schneider & Suomi, 1992) was to explore main aspects of infant neurobehavioral development: temperament, interactive behaviors, and neuromotor development (see also Bard, 2005, for a comparative perspective). For the purpose of this study, we focused on more specific categories of neurobehavioral development: coordinated reaching–grasping, motor maturity of posture, grasping reflexes, attention to visual stimuli, and emotional behavior. The choice of these subclusters is justified by the fact that they reflect the functioning of well-established cortical and subcortical neural motor circuit. For example, motor maturity of intentional movements, such as reaching–grasping, is related to the development of cortical parieto-frontal circuit (see Rizzolatti & Luppino, 2001). In contrast, posture and muscle tone are related to the maturation of spinal and subcortical structures. Other behaviors, such as auditory responses or tactile responses have not been considered in this study as they were aimed to explore the maturation of the sensory systems and thus outside the scope of the present investigation. Ratings were based on scales ranging from 0 to 2.
Coordinated reaching–grasping was scored by rating the quality of movements and fine hand motor manipulation, and during the visual presentation of a small object in front of the infant. Motor maturity of posture was scored by rating muscle tonus-prone of the head, response speed, body righting, arm traction, and labyrinth righting. Grasping reflexes were scored by rating infant response to a tactile stimulus placed on the palm. The capacity of infants to visually attend to stimuli was assessed by rating their orientation to visual stimuli presented in different positions of the visual space, by rating their ability to visually track moving stimuli, and by evaluating their duration of looking at the stimuli. For emotional behavior, we assessed the infants’ irritability to environmental stimuli and noises, and the levels of fearfulness. For a complete description of assessed behaviors, see Schneider and Suomi (1992). These assessments were part of a well-established protocol in nursery-reared infants in this primate facility, and scorers were not informed of any relation of these assessments to neonatal imitation abilities.
Statistical Analysis
Neonatal Imitation Analysis
In each condition, we assessed whether the behavior that matched the behavior provided by the demonstrator (target behavior) was performed by the infant more frequently during stimulus periods than during baseline, using paired t tests on each testing day. To assess the specificity of the infant response, we also compared the frequency of the nonmatched behavior (lipsmacking in the TP condition and tongue protrusion in the LPS condition) during the stimulus period with that displayed during baseline using paired t tests.
Based on their consistency of behavioral responses toward the stimuli, we categorized each animal as either imitator or nonimitator. If an infant displayed the matched gesture with a frequency above the baseline for more than 50% of the sessions in which it was tested (maximum of four sessions), it was classified as imitator. Those infants that failed to match the gesture with a higher frequency than in baseline in more than 50% of sessions were classified as nonimitator. We then compared the differential behavioral scored obtained by the two categories of infants on each postpartum day with two-tailed t tests. Individuals classified as imitator in the TP condition were not necessarily imitators in the LPS condition. Thus, the following analyses have been carried out independently for imitators and nonimitators for both the TP and LPS conditions.
Neurobehavioral Analysis in Infant Imitators and Nonimitators
To determine if there were significant differences between imitators’ and nonimitators’ development of neurobehavioral skills, we applied a repeated measure analysis of variance with imitation (imitator and nonimitator) as a between-subjects factor and postpartum day of testing (Day 7, Day 14, Day 21, and Day 30) as a within-subject factor. When significant effects were found, subsequent post hoc (Fisher tests) were applied to explore differences in developmental changes between the two groups. Further t tests were applied to compare the neurobehavioral scores of imitators and nonimitators on each testing day.
Results
Neonatal Imitation Data
In the TP condition, the frequency of tongue protrusion in the stimulus period was significantly higher than in the baseline period on postpartum Day 3 (t = −2.16, p < .05), Day 5 (t = −2.84, p < .05), and Day 7 (t = −4.41, p < .001) but not on Day 1. There was a trend for increases in LPS in response to the tongue protrusion stimulus on Day 3 (t = −1.85, p < .08) but not on any other day. There were no correlations between duration of looking at model and increases in TP on any test day (Pearson’s correlations: all ps > .05).
In the LPS condition, the frequency of lipsmacking in the stimulus period was significantly higher than in the baseline on Day 3 (t = −3.2, p < .005), Day 5 (t = −2.14, p < .05), and Day 7 (t = −2.4, p < .05). On Day 1, this increase approached significance (t = −1.93, p < .07). This increase was specific, as TP did not increase in response to the LPS stimulus on any day (all ps > .1).
Evaluation of the consistency of imitative responses led to 20 infants being categorized as imitators (imi) and 16 as nonimitators (nonimi) in the TP condition. At the same time, we categorized 21 as imitators and 15 as nonimitators in the LPS condition. Fourteen infants were imitators in both conditions TP and LPS. Five infants were discarded from analysis because imitative abilities were assessed only on one testing day. Another 3 infants were discarded from the analysis of birth development as they were born from cesarean section.
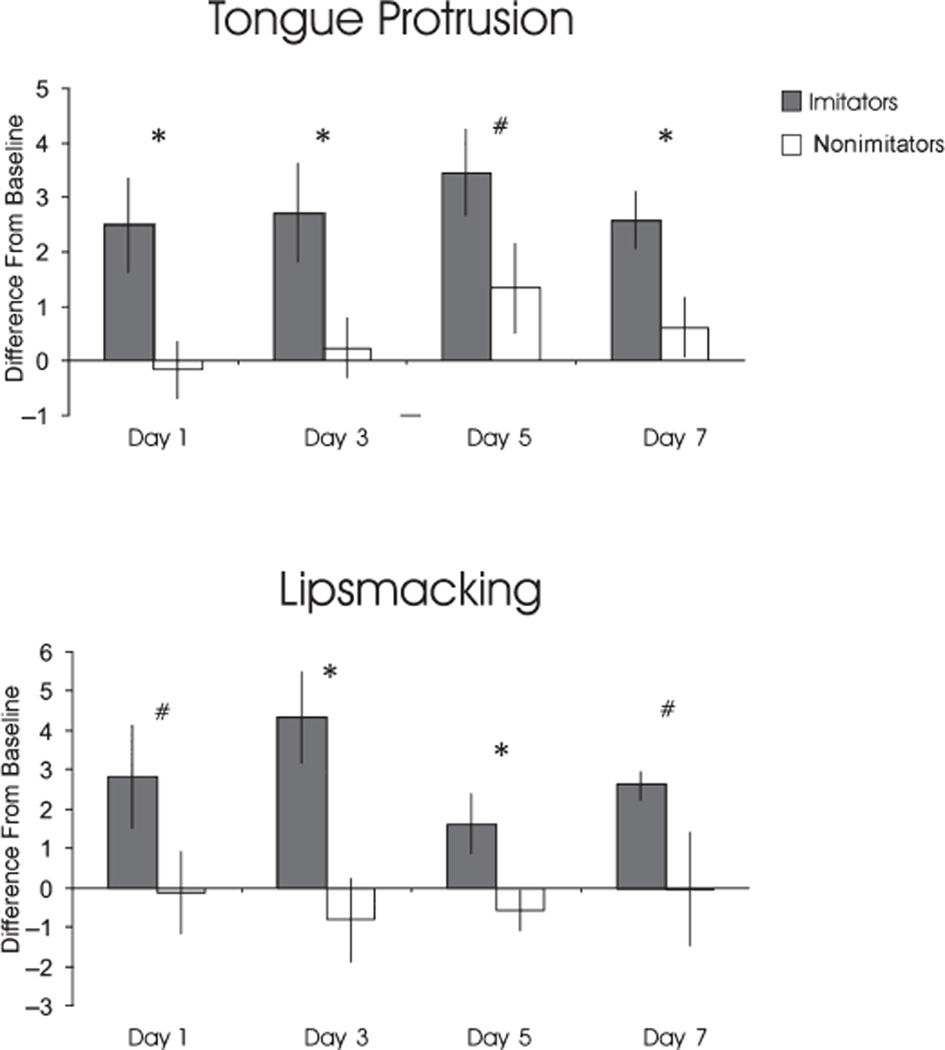
In the TP and LPS conditions, we compared imitative performance of imitators and nonimitators (one-tailed t test). Figure 1 summarizes the results of this analysis. In the TP condition, the imitation score of imitators was significant higher than those of nonimitators on Day 1 (t = 2.8, p < .001), Day 3 (t = 2.34, p < .05), and Day 7 (t = 2.31, p < .05). On Day 5, this difference was close to reaching significance (t = 1.6, p < .075). In the LPS condition, imitation scores of imitators were significantly higher than those of nonimitator on Day 3 (t = 3.0, p < .001) and Day 5 (t = 2.06, p < .05). On Days 1 and 7, this difference approached significance (d1: t = 1.38, p < .09; d7: t = 1.40, p < .09).
Figure 1.
The imitative averaged scores (difference of frequency between stimulus period and baseline) ± SEM of infant macaques tested on different postpartum days. Infant macaques have been categorized into two groups, imitators and nonimitators, according to their consistent imitative response toward the model on different testing days. Top: Infant tongue protrusion in response to the experimenter tongue protrusion gesture. Bottom: Infant lipsmacking in response to the experimenter lipsmacking gesture.
Note. *Significant difference between imitator and nonimitators (.05 < p < .01). #Difference between imitator and nonimitators approached statistical significance (.07 < p < .09).
Developmental Changes in Neurobehavioral Parameters
Infants Categorized Based on the Capacity to Imitate Tongue Protrusion
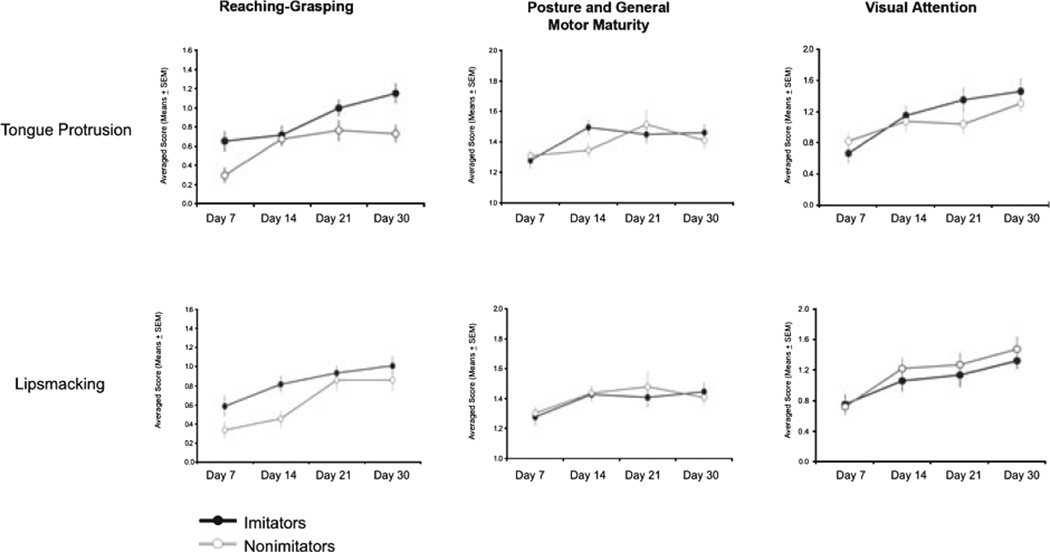
Results relative to the infant development of reaching–grasping, motor maturity, and visual attention are reported on Figure 2 (figures on the top).
Figure 2.
The frequency scores of infant macaques obtained during neurobehavioral assessment in the 1st month of life. Infants have been assigned to one of the two categories (imitators or nonimitators) according to their consistent imitative responses during the 1st week of life. Top and bottom graphs refer to the neurobehavioral scores obtained by infants categorized according to their neonatal imitative responses to tongue protrusion and lipsmacking gestures, respectively. Black dots indicate imitators and white dots indicate nonimitators.
Reaching–grasping
There was a significant main effect for postpartum day, F(1, 35) = 32.86, p < .001, and imitation ability, F(1, 35) = 7.32, p < .01, but no interaction. Imi obtained higher scores than nonimi in reaching–grasping on Day 7 (t = 2.41, p < .05), Day 21 (t = 1.82, p < .08), and Day 30 (t = 2.96, p < .005).
Motor maturity
Parameters of motor maturity indicate a significant improvement with age, F(1, 35) = 10.31, p < .005, but no effect for imitation and no interaction.
Visual attention
The capacity to attend to visual stimuli increased with age F(1, 35) = 19.56, p < .0001. There was no effect for imitative ability, and no interaction.
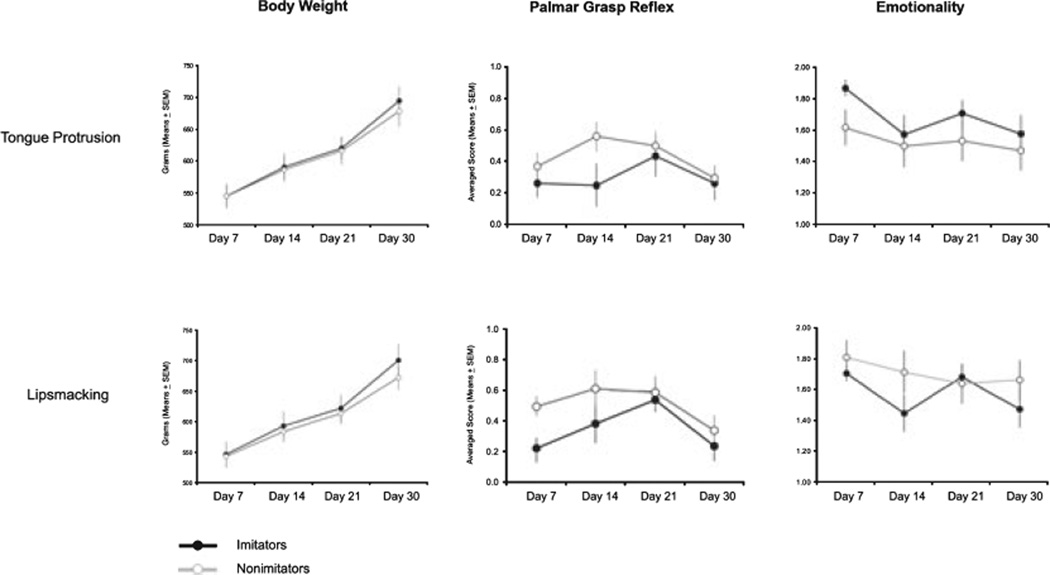
Results relative to the infant development of weight, palmar reflex, and emotionality are reported on Figure 3 (figures on the top).
Figure 3.
The weight development and frequency scores of infant macaques obtained during neurobehavioral assessment in the 1st month of life. For other details, see caption of Figure 2.
Weight
Infants’ weight increased with age, F(1, 32) = 178.86, p < .001. However, there was no significant effect for imitation, and no interaction between imitation and weight. Imi and nonimi did not differ in their weight increase in the 1st month of life.
Palmar reflex
There was a significant main effect for imitation, F(1, 35) = 4.39, p < .05, but not for age, and no interaction. In particular, nonimi had higher scores on palmar reflexes than imi on post-partum Day 14 (t = −2.04, p < .05).
Emotional behavior
There was no significant main effect or interaction.
Infants Categorized Based on the Capacity to Imitate Lipsmacking
Results relative to the infant development of reaching–grasping, motor maturity, and visual attention are reported on Figure 2 (figures on the bottom).
Reaching–grasping
There was a significant main effect for postpartum day, F(1, 35) = 33.14, p < .001, and imitation ability, F(1, 35) = 3.98, p < .055, but no interaction. More specifically, imi had higher scores than nonimi in reaching–grasping on Day 14 (t = 2.991, p < .005) and marginally on Day 7 (t = 1.62, p < .1).
Motor maturity
There was a main effect for age, F(1, 35) = 5.09, p < .005 but not for imitation ability, and no interaction.
Visual attention
The capacity to attend at visual stimuli increased with age, F(1, 35) = 9.17, p < .0001, but was not affected by imitative ability. There was no interaction.
Results relative to the infant development of weight, palmar reflex, and emotionality are reported on Figure 3 (figures on the bottom).
Weight
The weight of all infants increased with age, F(1, 32) = 302.65, p < .0001. There was no main effect for imitation, and no interaction.
Palmar reflex
Statistical analysis revealed a main effect for imitation ability, F(1, 35) = 4.46, p < .05. In particular, nonimi had higher scores on palmar reflexes than imi on postpartum Day 7 (t = −2.26, p < .05).
Emotional behavior
There was a tendency for emotionality to decrease with age, F(1, 35) = 2.57, p < .08. Emotional behavior did not differ between imi and nonimi, and there was no interaction.
Discussion
The results of this study confirm previous findings on neonatal imitation in macaques (Ferrari et al., 2006). Compared to baseline responses, newborn rhesus macaques increased the frequency of tongue protrusion and lipsmacking after having observed the same behavior being performed by a human experimenter. Our data show that this increase is gesture specific, and thus the infants’ behavioral response cannot be explained in terms of general arousal activation caused by the observation of human facial movement.
Some differences between the present study and the previous study require consideration. In the present investigation, the temporal window for neonatal imitation was wider than in the previous study. Infant macaques imitated facial gestures throughout the 1st week of life and not only for the first few days as previously reported (Ferrari et al., 2006). It is noteworthy that we previously observed (Ferrari et al., 2006) that some infants were still imitating the model at Day 7, even though the result was not significant at a population level. Differences in the experimental procedure might partly explain this difference. For example, in this study, the second cohort was assessed with only one condition per session, which reduced testing time, thereby minimizing attentional effort. As a result, it is possible that infants were more responsive to the stimuli. Although we did not systematically study the effects of prolonged stimulus exposure on infant attention, from our personal observation we noticed that infant attention to a stimulus is highly variable from individual to individual and that after 3 min of stimulus exposure, the interest of the infant to the stimulus appeared significantly decreased.
An alternative explanation, not necessarily exclusive, is that infants received repeated stimulation and thus could have been more stimulated to respond to the experimenter’s gesture. In fact, they were tested on Day 5 and thus received an additional exposure to the stimulus compared to the previous study in which infants had not been tested on Day 5. Moreover, as mentioned earlier, infants were tested only for a brief time period in which their attention could probably be well focused on the stimulus.
By comparing previous data with the present results, it seems plausible to think that neonatal imitation could emerge very early as a stereotype-like response to specific class of stimuli. However, very soon in development it clearly exhibits sensitiveness to environmental changes, suggesting that it can be subjected to modulations according to the context and the type of stimulation that the infant receives. This process would be particularly suitable for mother–infant relationship regulation.
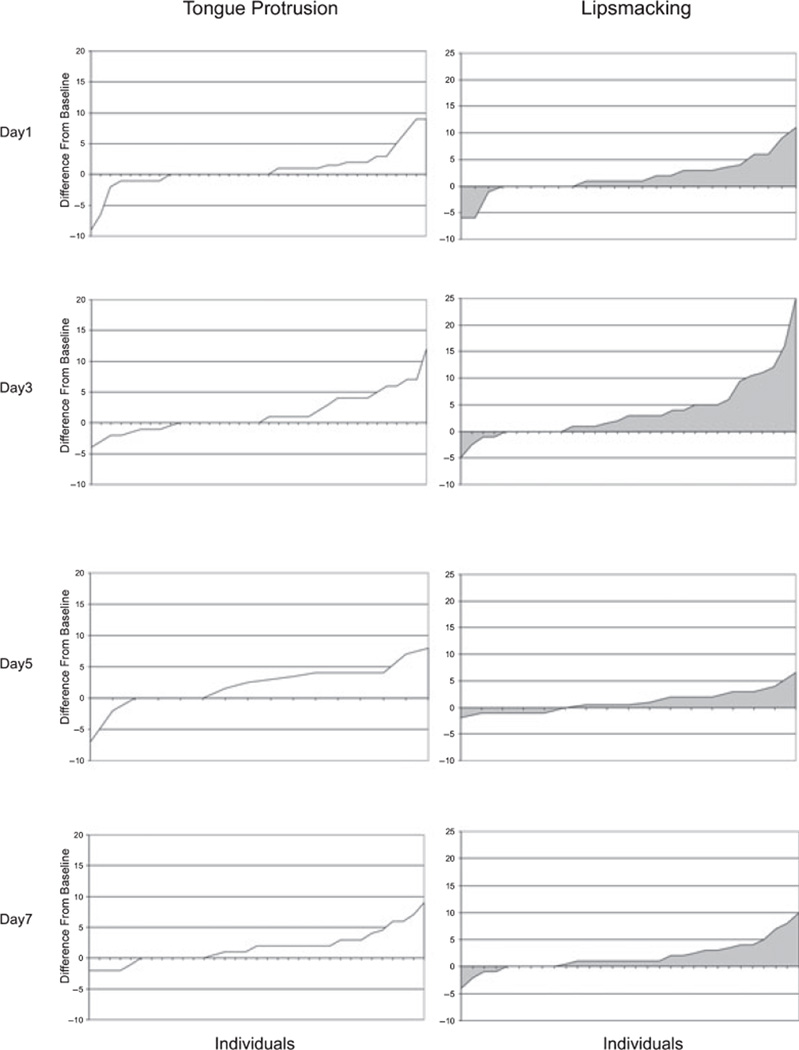
An interesting result from our investigation was the finding of significant interindividual variability of newborns’ responses to facial stimuli (see Figure 4). Some individuals were consistently imitating, whereas others were consistently not imitating the model on all testing days. It is also worth noting that more than one third of the infants (about 38%) were categorized as imitators for both lipsmacking and tongue protrusion gestures. As infants were tested and reared under identical circumstances, it is likely that these differences could be partly attributed to basic genetic differences in temperament. Other aspects of macaque behavior and neurobiology also tend to be consistent during development and, in some cases, have been linked to an overall genetic profile (Barr et al., 2003; Ferrari, Palanza, Parmigiani, de Almeida, & Miczek, 2005, for a review). Even though we did not assess the infants’ genetic background, the consistent behavioral response of imitators and nonimitators suggests that at birth their neurocognitive mechanisms aimed at capturing social environment are already differently sensitive and responsive to social cues.
Figure 4.
Each graph depicts the neonatal imitative scores of each individual. Each tick on the x-axis represents an individual. A negative score indicates that a behavior was observed more frequently during baseline; a positive score indicates that a behavior was observed more frequently during the stimulus period. The scores have been ordered from left to right in ascending order. Each tic represents a single infant. Note that an infant that had an imitative score on a specific day is positioned on a specific place along the abscissa. The same infant is not necessarily represented in the same position in the graph portraying a different day. Left and right graphs refer to the imitative scores obtained by infants categorized according to their neonatal imitative responses to tongue protrusion and lipsmacking gestures, respectively.
A core result of our research is that the ability to imitate was predictive of later behavioral–cognitive development, particularly coordination of reaching and grasping movements. Alternative explanations for this difference, such as differences in weight, postural maturity or visual attention, can be ruled out by the present results. Comparison of neonatal imitation data from other primate species further highlights the lack of relation between neonatal imitation and motor and visual development. Human and chimpanzee newborn imitate basic facial gestures during the first 2 months of life, a time when infants of both species are still altricial and highly dependent on their mothers. Independent locomotor activity is achieved only after neonatal imitative capacities have already disappeared (Johnson & Blasco, 1997). In contrast, while still being highly dependent on their mothers, infant macaques imitate within a period when they also start to have basic, although unstable, independent locomotor movements. In addition, their head posture and visual orientation capacities are relatively well developed at birth compared to those of humans and chimpanzees (Schneider & Suomi, 1992). Assuming that neonatal imitation is based on the same neural mechanisms in all three species, motoric and imitative capabilities may, but not necessarily have to, develop in parallel, which confirms the view that neonatal imitation is underpinned by neural mechanisms that are relatively independent from those controlling posture, visual, and locomotor development.
In the light of our findings, two basic questions need to be answered: (a) why do imitators differ from nonimitators in reaching–grasping in the 1st month of life? and (b) what are the neural substrates that connect reaching–grasping with neonatal imitation?
To answer these questions, we first need to understand the neural basis of reaching and grasping from a developmental perspective. Reaching and grasping represent voluntary goal-directed movements that require complex visual processing and visuomotor coordination in space. The capacity to display skilled hand movements is highly dependent on the maturation of costicospinal tract (Galea & Darian-Smith, 1995; Lemon, 1999). At birth, macaques have substantial changes in the corticospinal tract that is involved in the control of arm movements. The myelination of the corticospinal axons, for example, is only completed after 36 months (Olivier, Edgley, Armand, & Lemon, 1997). However, voluntary control of goal-directed movements is not merely a problem of maturation of the corticospinal tract. To have an efficient visually guided hand, infants must coordinate gaze behavior with arm movements and proprioception, and integrate this information with space coding for localizing object and coordinate movements in space (Von Hofsten, 2004). This complex integration is likely to start in the 1st week of life, if not before (Bauer & Held, 1975; Held & Bauer, 1967, 1974). Elegant demonstrations of this early stage integration are the experiments with newborn macaques that were prevented to see the own arm at birth. Once tested at 35 days of age they experienced severe impairment in visually guided reaching and grasping despite their preserved capacity to grasp under tactile guidance (Held & Bauer, 1967).
A key role in processing and integrating visual and proprioceptive information with hand and arm movements is played by the posterior parietal and motor cortical areas (Fogassi & Luppino, 2005; Rizzolatti & Luppino, 2001). Parietal and motor areas in the monkey brain are constituted by several distinct areas that are reciprocally anatomically connected (Rozzi et al., 2006) and form parietal–frontal circuits that work in parallel for sensorimotor transformations. Some of the main functions of these circuits are aimed at transforming visual information about object features and location into an appropriate action (Murata et al., 1997; Murata, Gallese, Luppino, Kaseda, & Sakata, 2000; Rizzolatti, Luppino, & Matelli, 1998). Other circuits process specific visual information for matching action observation with the internal motor representation of the observed action (Fogassi et al., 2005; Rizzolatti & Luppino, 2001).
Among these circuits, the one that connects the macaque inferior parietal cortex (area PF–PFG) and part of the ventral premotor cortex, area F5, is particularly important. Neurophysiological studies with single neuron recording have shown that neurons in both these areas code specific hand and mouth actions (e.g., grasping, holding, biting). In each class, neurons might code specific aspect of the actions such as opening or closing of the hand or mouth or, in the case of the hand, the type of hand shaping (e.g., precision grip, whole hand prehension). Some of these neurons in the macaque premotor and parietal cortices belong to a particular class of visuomotor neurons named mirror neurons (Ferrari, Gallese, Rizzolatti, & Fogassi, 2003; Fogassi et al., 2005; Gallese, Fadiga, Fogassi, & Rizzolatti, 1996; Gallese, Fogassi, Fadiga, & Rizzolatti, 2002; Rizzolatti, Fadiga, Gallese, & Fogassi, 1996). Mirror neurons discharge both when the monkey executes a goal-directed action, with the hand or the mouth, and when it observes a similar action performed by another individual. In other words, mirror neurons are able to match an observed action by activating a cortical representation of that same action that has been hypothesized to give access to the meaning of the observed action (Gallese et al., 1996; Rizzolatti & Craighero, 2004).
Recently, it was found that the representation of actions is organized in chains of motor acts (Ferrari et al., 2006; Fogassi et al., 2005). In the parietal areas PFG/PF and the ventral premotor area F5, motor neurons code the same motor act (e.g., grasping) differently according the final goal of the action in which they are embedded (eating the object or placing it in a container), reflecting the intention of the monkey to grasp the object for eating or placing it. It has been proposed that one of the advantages of organization in chains is the facilitation of subsequent motor acts that belong to the same specific chain (e.g., reaching, grasping, bringing to the mouth, and biting a piece of food). Therefore, the activation of a node of a specific chain, for example, grasping a piece of food, preactivates the subsequent node of the chain, in this example bringing the food to the mouth. Interestingly, some action-constrained neurons have also mirror properties (Fogassi et al., 2005). They become active when the observed action is aimed at a specific goal (such as when an experimenter grasps food to eat but less so when grasping is aimed at placing the food in a container). It has been hypothesized that these neurons provide information not only about what another individual is doing (grasping) but also why he/she is doing it (to eat). Overall, these findings indicate that the neuronal chain organization present in premotor and parietal cortical areas is recruited not only at the motor level for pragmatic purposes (i.e., the control of grasping actions in space) but it has also an impact on other cognitive faculties such as understanding others’ behavior and intention and, most likely, imitation.
Our data show that a basic chain organization for reaching and grasping is present very early in infant macaques, with some individuals relying on better developed chains than others. The clumsiness of the movement at birth and the improvement with age suggest that these chains are part of an “open” developmental program that integrates several aspects of the infants’ sensori-motor system with practice and learning, possibly through a biologically plausible Hebbian learning dynamics (Chersi, Fogassi, Bonini, Rizzolatti, & Ferrari, 2007).
Taken together, our results are in agreement with the view that cortical motor organization in imitators is at a more advanced stage of development than in nonimitators. This view is also supported by the data showing that nonimitators have more developed palmar reflexes than imitators. In fact, it has been proposed that palmar reflexes tend to disappear with infant maturation of cortical motor areas (Herschkowitz, 2000; Paulson & Gottlieb, 1968). Thus, the difference between imitators and nonimitators in the palmar reflex would reflect the differential maturation of the motor cortex.
Our data also suggest that heightened responsiveness to imitate facial gestures is related to advanced development of premotor and parietal cortical areas that are involved in reaching–grasping. We hypothesize that neonatal imitation is connected to the neural system that matches an observed gesture with an internal representation of that gesture. According to this hypothesis, an infant’s facial gesture is activated by the observation of the same gesture performed by another individual because mirror neurons activity triggers a corresponding motor output in the parietal and premotor cortical areas. These same areas, as previously described, partly overlap with those involved in the coding of grasping actions (Rizzolatti et al., 2001). Although there are no studies on the neural basis of imitative behaviors in monkeys (in the sense of copying an action already present in the own motor repertoire), it has been hypothesized that mirror neurons are responsible for neonatal imitative capacities in macaques (Ferrari et al., 2006). There is now clear evidence of a mirror neuron system in humans and that it not only allows an individual to understand others’ actions but also to imitate simple movements and complex actions (Buccino et al., 2005; Iacoboni et al., 1999; see also Rizzolatti et al., 2002). These studies show that a circuit involving the posterior parietal cortex, the inferior frontal gyrus, and the premotor cortex adjacent to it (the human mirror neuron system; see Rizzolatti & Craighero, 2004, for monkey–human homology) activates both when a subject is observing a simple movement (lifting a finger) or a complex action (playing a guitar chord), and when the subject copies that same action. Therefore, it is likely that the differences in infant imitative abilities reflect a difference in the development of the mirror neuron circuit present in the premotor and parietal cortical areas of the infant macaques.
A delay in the development of motor chains does not necessarily imply that individuals have cognitive deficits, but might suggest that some individuals may be delayed in the achievement of important cognitive landmarks. Even though this hypothesis requires empirical testing, a recent study points to a connection between deficits in motor action chains and impairments in social-cognitive skills (Cattaneo et al., 2007). High-functioning autistic children show impairments in motor chains activation recorded with electromyography from arm and leg muscles while performing grasping motor acts for different goals. Moreover, they differ in their motor chain activation from typically developing children while observing the same action sequences performed by another individual, suggesting that their mirror neuron system for understanding others’ intentions is impaired (Cattaneo et al., 2007). This finding is in agreement with previous reports showing that autistic children have motor disturbances both during planning and execution of reaching–grasping actions (Mari, Castiello, Marks, Marraffa, & Prior, 2003).
The connection between a neural matching system, mirror neurons, and autism has been documented by several other investigations (Iacoboni & Dapretto, 2006; Oberman et al., 2005; Williams, Whiten, Suddendhorf, & Perrett, 2001). These findings suggest that significant deficits in social-cognitive functions could be linked an inability to match others’ actions with their own actions and to impairments in the cortical motor organization of goal-directed actions. Whether this inability could be present at birth is a matter for further investigations.
To the best of our knowledge, there are few longitudinal studies in humans looking at infant imitative response at birth and the development of behavioral and cognitive functions. One study looked at neonatal imitation, imitation, and temperament in 12-month-old children but found that the latter two did not correlate with neonatal imitative abilities (Heimann et al., 1989). Our data seem to open a new perspective in the field of cognitive development and indicate that basic social skills at birth may predict future behavioral developments.
Acknowledgments
This research has been supported by the Division of Intramural research, National Institute of Child Health and Human Development, NIH, from the Cofin 2007 and the European Program Neurocom n.12738, 2005–2008.
Contributor Information
Pier Francesco Ferrari, Università di Parma Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Annika Paukner, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Angela Ruggiero, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Lisa Darcey, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Sarah Unbehagen, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Stephen J. Suomi, Eunice Kennedy Shriver National Institute of Child Health and Human Development
References
- Abravanel E, Sigafoos AD. Exploring the presence of imitation during early infancy. Child Development. 1984;55:381–392. [PubMed] [Google Scholar]
- Anisfeld M. Only tongue protrusion modeling is matched by neonates. Developmental Review. 1996;16:149–161. [Google Scholar]
- Bard KA. Emotions in chimpanzee infants: The value of a comparative developmental approach to understand the evolutionary origins of emotions. In: Nadel J, Muir D, editors. Emotional development. Oxford, UK: Oxford University Press; 2005. pp. 31–60. [Google Scholar]
- Bard KA. Neonatal imitation in chimpanzees (Pan troglodytes) tested with two paradigms. Animal Cognition. 2007;10:233–242. doi: 10.1007/s10071-006-0062-3. [DOI] [PubMed] [Google Scholar]
- Bard KA, Russell CL. Evolutionary foundations of imitation: Social cognitive and developmental aspects of imitative processes in non-human primates. In: Nadel J, Butterworth G, editors. Imitation in infancy. Cambridge, UK: Cambridge University Press; 1999. pp. 89–123. [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, et al. Early experience and rh5-HTTPLR genotype interact to influence social behavior and aggression in nonhuman primates. Genes, Brain and behavior. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Bauer J, Held R. Comparison of visually guided reaching in normal and deprived infants monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 1975;4:298–308. doi: 10.1037//0097-7403.1.4.298. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, et al. Neural circuits underlying imitation learning of hand actions: An event-related fMRI study. Neuron. 2005;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Cattaneo L, Fabbri-Destro M, Boria S, Pieraccini C, Monti A, Cossu G, et al. Impairment of actions chains in autism and its possible role in intention understanding. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17825–17830. doi: 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chersi F, Fogassi L, Bonini L, Rizzolatti G, Ferrari PF. Modeling intentional neuronal chains in parietal and premotor cortex [Abstract] Society for Neuroscience Abstracts. 2007;636 [Google Scholar]
- Dettmer AM, Novak MA, Meyer JD, Ruggiero A, Suomi SJ. Surrogate mobility and orientation affect the early neurobehavioral development of infant rhesus macaques (Macaca mulatta) Developmental Psychobiology. doi: 10.1002/dev.20296. in press. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. European Journal of Neuroscience. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Palanza P, Parmigiani S, de Almeida RMM, Miczek KA. Serotonin and aggressive behavior in rodents and nonhuman primates: Predispositions and plasticity. European Journal of Pharmacology. 2005;526:259–273. doi: 10.1016/j.ejphar.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biology. 2006;4:1501–1508. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field TM, Woodson R, Greenberg R, Cohen D. Discrimination and imitation of facial expression by neonates. Science. 1982;218:179–181. doi: 10.1126/science.7123230. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Chersi F, Gesierich B, Rozzi S, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G. Motor functions of the parietal lobe. Current Opinion in Neurobiology. 2005;15:626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Fontaine R. Imitative skills between birth and six months. Infant Behavior and Development. 1984;7:323–333. [Google Scholar]
- Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cerebral Cortex. 1995;5:518–540. doi: 10.1093/cercor/5.6.518. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fogassi L, Fadiga L, Rizzolatti G. In: Common mechanisms in perception and action: Attention and performance. Prinz W, Hommel B, editors. Vol. 29. Oxford, UK: Oxford University Press; 2002. pp. 334–355. [Google Scholar]
- Heimann M, Nelson KE, Schaller J. Neonatal imitation of tongue protrusion and mouth opening: Methodological aspects and evidence of early individual differences. Scandinavian Journal of Psychology. 1989;30:90–101. doi: 10.1111/j.1467-9450.1989.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Held R, Bauer JA. Visually guided reaching in infant monkeys after restricted rearing. Science. 1967;155:718–720. doi: 10.1126/science.155.3763.718. [DOI] [PubMed] [Google Scholar]
- Held R, Bauer JA. Development of sensorially-guided reaching in infant monkeys. Brain Research. 1974;71:265–271. doi: 10.1016/0006-8993(74)90970-6. [DOI] [PubMed] [Google Scholar]
- Herschkowitz N. Neurological bases of behavioural development in infancy. Brain and Development. 2000;22:411–416. doi: 10.1016/s0387-7604(00)00185-6. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Review Neuroscience. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: The cortical mechanisms of visuomotor transformation. Trends in Neurosciences. 1995;18:314–320. [PubMed] [Google Scholar]
- Johnson CP, Blasco PA. Infant growth and development. Pediatrics in Review. 1997;18:224–242. doi: 10.1542/pir.18-7-224. [DOI] [PubMed] [Google Scholar]
- Legerstee M. The role of person and object in eliciting early imitation. Journal of Experimental and Child Psychology. 1991;51:423–433. doi: 10.1016/0022-0965(91)90086-8. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Neural control of dexterity: What has been achieved? Experimental Brain Research. 1999;128:6–12. doi: 10.1007/s002210050811. [DOI] [PubMed] [Google Scholar]
- Mari M, Castiello U, Marks D, Marraffa C, Prior M. The reach-to-grasp movement in children with autism spectrum disorder. Philosophical Transactions of the Royal Society of London: Series B. Biological Sciences. 2003;358:393–403. doi: 10.1098/rstb.2002.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Early infant imitation within a functional framework: The importance of person identity, movement, and development. Infant Behavior and Development. 1992;15:479–505. doi: 10.1016/0163-6383(92)80015-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. Journal of Neurophysiology. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. Journal of Neurophysiology. 2000;83:2580–2601. doi: 10.1152/jn.2000.83.5.2580. [DOI] [PubMed] [Google Scholar]
- Myowa-Yamakoshi M, Tomonaga M, Tanaka M, Matsuzawa T. Imitation in neonatal chimpanzees (Pan troglodytes) Developmental Science. 2004;7:437–442. doi: 10.1111/j.1467-7687.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EM, Ramachandran ES, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Olivier E, Edgley SA, Armand J, Lemon RN. An electrophysiological study of the postnatal development of the corticospinal system in the macaque monkey. Journal of Neuroscience. 1997;17:267–276. doi: 10.1523/JNEUROSCI.17-01-00267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson G, Gottlieb G. Development reflexes: The reappearance of foetal and neonatal reflexes in aged patients. Brain. 1968;91:37–52. [Google Scholar]
- Rizzolatti G, Craighero L. The mirror neuron system. Annual Review in Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. From mirror neurons to imitation: Facts and speculations. In: Meltzoff AN, Prinz W, editors. The imitative mind. Development, evolution, and brain bases. Cambridge, UK: Cambridge University Press; 2002. pp. 247–265. [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Neuroscience Reviews. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: New concepts. Electroencephalography and Clinical Neurophysiology. 1998;106:283–296. doi: 10.1016/s0013-4694(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, et al. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cerebral Cortex. 2006;16:1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- Ruppenthal GC, Arling GL, Harlow HF, Sackett GP, Suomi SJ. A 10-year perspective of motherless-mother monkey behavior. Journal of Abnormal Psychology. 1976;85:341–349. doi: 10.1037//0021-843x.85.4.341. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): Developmental changes, behavioural stability, and early experience. Infant Behavior and Development. 1992;15:155–177. [Google Scholar]
- Suomi SJ. Attachment in rhesus monkey. In: Cassidy J, Shaver PR, editors. Handbook of attachment. New York: The Guilford; 1999. pp. 181–197. [Google Scholar]
- Towen BC. The neurological development of prehension: A developmental neurologists view. International Journal of Psychophysiology. 1995;19:115–127. doi: 10.1016/0167-8760(94)00080-x. [DOI] [PubMed] [Google Scholar]
- Von Hofsten C. An action perspective on motor development. Trends in Cognitive Sciences. 2004;8:266–272. doi: 10.1016/j.tics.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendhorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Review. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]