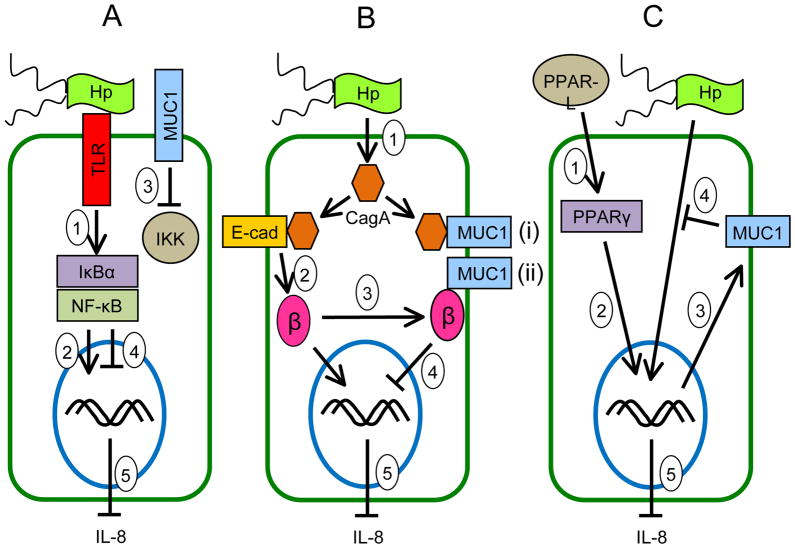
Figure 4.
Schematic illustration depicting the possible molecular mechanisms of the anti-inflammatory activities of MUC1 in gastric epithelial cells during H. pylori infection. (A) Inhibition of NF-κB signaling: H. pylori binds to cell surface receptors (e.g. Toll-like receptors, TLRs) that activate NF-κB following phosphorylation of IκBα by IKK (step 1). NF-κB enters the nucleus to activate proinflammatory (IL-8) gene expression (step 2). MUC1 blocks the ability of IKK to phosphorylate IκBα (step 3), thus inhibiting NF-κB nuclear translocation (step 4) and blocking IL-8 production (step 5). (B) Inhibition of β-catenin signaling: H. pylori delivers CagA into the cytosol (step 1). CagA binds to E-cadherin (step 2), thereby releasing β-catenin that translocates into the nucleus to activate proinflammatory (IL-8) gene expression. MUC1 binds to β-catenin (step 3) released by CagA, thus inhibiting its nuclear translocation (step 4) and blocking IL-8 production (step 5). (C) PPARγ-dependent inhibition: Endogenous PPAR-γ ligands (PPAR-L) activate PPARγ (step 1). PPARγ enters the nucleus (step 2) to activate MUC1 gene expression (step 3). MUC1 protein inhibits the H. pylori-driven NF-κB and/or β-catenin pathways (step 4), thus blocking IL-8 production (step 5).