Abstract
Introduction
The severe need for constructing replacement tissues in organ transplanta-tion has necessitated the development of tissue engineering approaches and bioreactors that can bring these approaches to reality. The inherent limitations of conventional bioreactors in generating realistic tissue constructs led to the devise of the microgravity tissue engineering that uses Rotating Wall Vessel (RWV) bioreactors initially developed by NASA.
Methods
In this review article, we intend to highlight some major advances and accomplishments in the rapidly-growing field of tissue engineering that could not be achieved without using microgravity.
Results
Research is now focused on assembly of 3 dimensional (3D) tissue fragments from various cell types in human body such as chon-drocytes, osteoblasts, embryonic and mesenchymal stem cells, hepatocytes and pancreas islet cells. Hepatocytes cultured under microgravity are now being used in extracorporeal bioartificial liver devices. Tissue constructs can be used not only in organ replacement therapy, but also in pharmaco-toxicology and food safety assessment. 3D models of vari-ous cancers may be used in studying cancer development and biology or in high-throughput screening of anticancer drug candidates. Finally, 3D heterogeneous assemblies from cancer/immune cells provide models for immunotherapy of cancer.
Conclusion
Tissue engineering in (simulated) microgravity has been one of the stunning impacts of space research on biomedical sciences and their applications on earth.
Keywords: Tissue Engineering, Aerospace Medicine, Bioreactors, Microgravity
Introduction
Vacanti et al introduced tissue engineering based on synthetic biodegradable polymer scaffolds in 1988 for potential replacement of missing or defective cartilage. Tissue engineering/regenerative medicine has the ultimate goal of generating functional 3D constructs which can be utilized as replacement organs with normal function, or serve for in vitro study of drug toxicity, safety and efficacy (Unsworth and Lelkes 1998, Langer 1997). So far, three principal approaches have been followed in tissue engineering: I; direct implantation of freshly isolated or cultured cells, II; in situ tissue regeneration, and III; assembly of cells and scaffolds in vitro (Korossis et al 2005). Novel model tissue engineering systems have two features: I; a biodegradable scaffold that determines the final shape and dimension of the constructs, and II; the culture environment that provides essential nutrients and appropriate mixing which will ensure a uniform cell seeding and proliferation (Freed and Vunjak-Novakovic 1997b).
Homotypic or heterotypic 3D multicellular spheroids provide a more natural cellular differentiation than 2D monolayer cultures and show improved mimicry of the behavior and function of actual tissues (Hoffman 1993). When spheroids are cultured in conventional Petri-dishes or bioreactors, the restricted nutrient and oxygen diffusion into the spheroids results in a hypoxic, necrotic center in constructs larger than 1 mm in size (Sutherland et al 1986) which limits the functional properties of the constructs. Microgravity has advanced the field of tissue engineering by facilitating diffusion of nutrients and oxygen into these spheroids and thus creating constructs devoid of necrotic centers (Unsworth and Lelkes 1998). Under microgravity conditions, aggregation of cells is also enhanced by induction of differentiative cellular signaling. These issues have led to achieving constructs larger than those engineered in conventional bioreactors or 2D cultures (Unsworth and Lelkes 1998).
In this review article, we will discuss the development of microgravity bioreactors along with cartilage and bone tissue engineering under microgravity. Advances in pancreas and liver tissue engineering, their potential applications in treatment of diabetes and acute liver failure and the important role of tissue engineering in cancer research and pharmaco-toxicology are also discussed.
Microgravity bioreactors for tissue engineering
Bioreactors are biomechanically active simulation systems that use mechanical means to influence biological processes. Bioreactors can contribute to in vitro formation of tissues by providing and tightly controlling the biochemical and physical regulatory signals to cells. The mechanical stimulation by bioreactor can encourage cells to differentiate (Altman et al 2002) and produce extracellular matrix more quickly (Carver and Heath 1999). Bioreactors provide tissue cultures by required nutrients and gases and facilitate nutrient transport to and waste transport from the tissue. Bioreactors and especially microgravity bioreactors can maintain a spatially-uniform cell distribution throughout the tissue engineering scaffold (Partap et al 2010, Goldstein et al 2001, Yu et al 2004).
Space research contributed to the expanding field of tissue engineering by combining cell culture and microgravity. Years ago, it was recognized that microgravity might benefit tissue engineering by promoting cell-cell association while minimizing turbulence and shear stress. Indeed, cells in suspension tend to aggregate when exposed to microgravity (Hymer et al 1996, Dintenfass 1986). Microgravity promotes co-location of cells and initiation of differentiative cellular signaling via induction of specialized cell adhesion molecules and extracellular matrix proteins. These in turn, may lead to establishment of 3D tissue constructs (Freed and Vunjak-Novakovic 1995). Microgravity has also been shown to potentiate stem cell proliferation while sustaining their capability for differentiation (Yuge et al 2006), which can be of utmost importance in tissue engineering.
First examples of microgravity devices fabricated by researchers at NASA were Slow-Turning Lateral Vessel (STLV) and the High Aspect Ratio Vessel (HARV) (EL-Haj AJ and Cartmell 2010, Martin and Vermette 2005). RWV bioreactor was introduced for studying tissue generation and cell behavior under microgravity (Schwarz et al 1992). In a RWV bioreactor, two concentric cylinders exist: an inner cylinder from silicone rubber that is stationary and is meant for gas exchange and the outer cylinder capable of rotating at a constant angular speed. Rotation of the vessel provides an upward hydrodynamic drag force against the downward gravitational force. When the gravitational forces are balanced with centrifugal forces, a microgravity-like culture condition is created within the cylinders in the annular space (Partap et al 2010). With gradual increase in the size of the tissues in bioreactor, the rotation speed must increase to balance the gravitational force and maintain the scaffold in suspension (Partap et al 2010, Kwon et al 2008). Media can be exchanged through a fluid pump. RWV bioreactor is now commercially available from Synthecon in USA (Houston, Texas) and from Cellon in Europe (Luxembourg). Fig. 1 shows a schematic representation of RWV bioreactor, which is often used in tissue engineering under, simulated microgravity.
Fig. 1 .
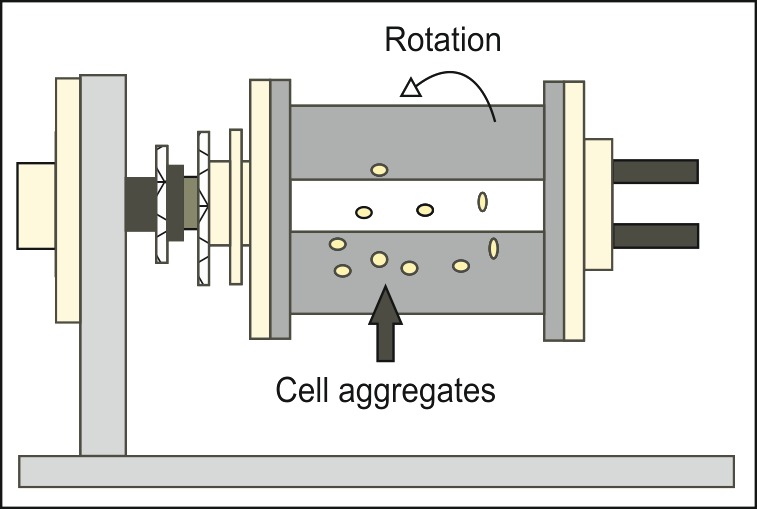
RWV microgravity bioreactor developed by NASA.
The fluid dynamics of RWV bioreactors allows for diffusion of oxygen and nutrients to the cell aggregates and results in tissue constructs devoid of necrotic cores (Hammond and Hammond 2001, Unsworth and Lelkes 2000). Shear stress can be harmful to the engineered tissue constructs. Recently, the safe range of microcarrier radius or tissue size to avoid shear stress in RWV bioreactors has been determined (Farrag 2009). In another attempt, appropriate operating parameters for a RWV bioreactor such as oxygen transport and consumption and optimal rotation speed were determined (Kwon et al 2008). In a numerical simulation, different parameters involved in a successful simulation of microgravity such as fluid shear, mass transport, collisions between microcarriers and between the microcarrier and walls of the cylinder, and the use of adequate and appropriate controls have been discussed (Ayyaswamy and Mukundakrishnan 2007). One of the limitations of RWV bioreactors is that when tissue engineering scaffolds with more density than the culture environment is used, cell aggregates fall to the bottom of the cylinder.
As first attempts of tissue engineering in microgravity, RWV bioreactors were used for formation of cartilaginous constructs composed of round cells, collagen and glycosaminoglycan, and cardiac tissue constructs contracting spontaneously and synchronously (Freed and Vunjak-Novakovic 1997b). In this study, constructs grown in microgravity had the highest fractions of regenerated tissue and glycosaminoglycan content (the component required for cartilage to endure compressive forces) compared to constructs grown in rotating bioreactors, turbulent mixers and Petri-dishes (Freed and Vunjak-Novakovic 1997b). However, 3D aggregates did not form in every case, e.g. the insect ovary cell line SF-9 did not aggregate in RWV bioreactor (Francis et al 1997). A good list of early examples of cells and tissues cultivated in (simulated) microgravity has been presented by Unsworth et al (1998).
Cartilage tissue engineering in microgravity
Chondrocyte aggregates have been generated on beads (Duke et al 1996), meshes (Hu and Athanasiou 2005) and novel porous biopolymers such as chitosan (Nettles et al 2002) in RWV bioreactors. Ohyabu et al reported the rapid regeneration of 3D large cartilaginous tissue from rabbit bone marrow cells (without a scaffold) using a RWV bioreactor (Ohyabu et al 2006). The same group succeeded to control the cartilage tissue shape from rabbit bone marrow cells by RWV bioreactor using a collagen sponge scaffold which enhanced the glycosaminoglycan content of the generated tissues and strengthened the compression strength of the product (Ohyabu et al 2009). The cartilaginous tissue aggregates formed without scaffold from bone marrow-derived cells using the RWV bioreactor were placed in critical osteochondral defects in rabbit femur and rapid regeneration of defects were reported (Yoshioka et al 2007). In an attempt to engineer rat articular cartilage articular chondrocytes were cultured on 3D macroporous poly(DL-lactic-co-glycolic acid) (PLGA) sponges under microgravity with chondrogenic medium (containing TGF-β1) which led to redifferentiation of rat chondrocytes and formation of hyaline-like rat cartilage (Emin et al 2008). In RWV bioreactor, a hyaline cartilage tissue, which possessed favorable morphological properties, was engineered from human bone marrow-derived cells (Sakai et al 2009).
However, cartilage constructs flown in space are mechanically inferior to constructs grown on earth while those built in RWV bioreactors are quite similar in both composition and mechanical strength to natural cartilage (Freed et al 1997a, Freed et al 1998). This inferior quality of space-flown cartilage is in line with the widely-known adverse effect of space on bone, cartilage and even muscles (Stamenkovic et al 2010, Rucci et al 2007, Nabavi et al 2011).
Bone tissue engineering in microgravity
RWV bioreactors have been used to generate microcarrier-based (Granet et al 1998, Botchwey et al 2001) and porous scaffold-based (Turhani et al 2005, Song et al 2006, Song et al 2008, Kyriakidou et al 2008) osteoblastic cell culture. HARV bioreactor has been used for bone tissue engineering using poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds (Lv et al 2008). 3D osteoblast cell cultures on bioceramic microspheres and degradable composite microspheres were obtained in RWV bioreactor (Qiu et al 1999, Qiu et al 2001). Bone tissue engineering has also proved promising with mesenchymal stem cells grown on mineralized PLGA scaffolds (Koc et al 2008). Undifferentiated embryonic stem cells were encapsulated within alginate hydrogels and cultured in a rotary cell culture microgravity bioreactor. The generated constructs displayed the morphological, phenotypic, mechanical and molecular properties of the osteogenic lineage (Hwang et al 2009). Bone constructs engineered by culturing bone marrow mesenchymal stem cells on ceramic bovine bone scaffolds in static flasks and in rotating vessels were transplanted into Sprague-Dawley rat cranial bone defects. The engineered bone constructs under dynamic culture were found to repair the defects better than static counterparts after 24 weeks of in vivo implantation (Jin et al 2010). 3D environments such as Rotary Cell Culture System (RCCS), enhances osteoblast cell aggregation and mineralization (Facer et al 2005). Osseous-like tissues were also engineered in small volumes from preosteoblasts cultured in RWV bioreactors (Schneider et al 2011). Hydrodynamic microgravity can thus modulate the composition, morphology, and function of the engineered bone (Song et al 2006).
Improved mass transfer in the microgravity bioreactor and appropriate scaffold material have been suggested as decisive factors in bone tissue engineering (Araujo et al 2010). Shear stress is also known to have a role in osteoblastic differentiation, mineralization and calcium deposition of stem cells and has been reviewed comprehensively along with bioreactors used in bone tissue engineering by Yeatte and Fisher (2011).
Liver tissue engineering in microgravity
3D assemblies of human liver cells (up to 3 cm long) were achieved in simulated microgravity. Bile duct-like structures, cohesive hepatocytes, complex stromal structures, reticulin fibers, bile canaliculi, and tight cellular junctions were identified in the 3D assemblies by electron microscopy (Khaoustov et al 1999). Later, simulated microgravity environment was shown to maintain key metabolic functions and promote aggregation of primary porcine hepatocytes (which are difficult to maintain in normal culture) (Dabos et al 2001). Low-shear modeled microgravity has also been shown to maintain morphology and differentiated functionality of primary porcine hepatocyte cultures which is hard to achieve in normal culture (Nelson et al 2010). Rat hepatocytes cultured initially as spheroids on culture plates and then transferred into HARV, retain cellular and physiological properties of the intact liver, including drug-metabolizing enzyme activities, plasma protein production, and long-term viability (Brown et al 2003).
Entrapment of hepatocyte spheroids in a hollow fiber bioreactor was hypothesized as a potential bioartificial liver (BAL) in 1995 (Wu et al 1995). In an attempt to design an extracorporeal BAL device (Innsbruck Bioartificial Liver or IBAL), Hochleitner et al designed a bioreactor containing aggregates of porcine hepatocytes grown under simulated microgravity. Cell culture was possible for at least 10 days in the device (Hochleitner et al 2005). IBAL was then tested in pigs through induction of fulminant hepatic failure. The survival of pigs was significantly prolonged by about 150% with IBAL treatment as compared to controls (Hochleitner et al 2006). Small human hepatocytes in rotary culture were then utilized to construct a prototype BAL support system, in which cells demonstrated high viability (90-95%), and thus proved promising in establishment of a fully autonomous BAL as a bridge to transplantation (Wurm et al 2009). Very recently, a simple dummy liver assist device was shown to prolong anhepatic survival in a porcine model of total hepatectomy (Thiel et al 2011).
Microgravity tissue engineering and diabetes
Xenogeneic islets have been considered for transplantation in patients with insulin-dependent diabetes mellitus (Thompson and Mandel 1990). Allogeneic islet transplants have been successfully used in diabetic recipients, but chronic immunosuppressive agents are needed to prevent the rejection of transplanted cells (Ryan et al 2002, Shapiro and Lakey 2000a, Shapiro et al 2000b). Microgravity not only enhances the survival or proliferation of beta islet cells (Song et al 2004a, Song et al 2004b), but also reduces their immunogenicity by depleting dendritic cells which express the class II MHC (Rutzky et al 2002). Besides, islets have a better morphological, insulin normalizing and secretory profile under microgravity (Hou et al 2009).
Pancreatic islets from neonatal pigs, and Sertoli cells from prepubertal rats co-cultured in simulated microgravity, have been shown to form insulin-secreting, Sertoli-enriched tissue constructs (Cameron et al 2001a), which have been suggested for long-term transplantation treatment of diabetes (Cameron et al 2001b). Han et al transplanted islets and Sertoli cell aggregates co-cultured under microgravity to streptozotocin (STZ)-induced diabetic rats (Han et al 2009). STZ is used to induce diabetes in rats (Ghaffari et al 2012). During the in vivo studies, the animals remained euglycemic and the Sertoli-islets cell aggregates did not elicit allogeneic transplantation rejection, reducing the need for immunosuppressive agents (Han et al 2009).
Microgravity tissue engineering for generation of model tissues
3D tissue models mimic specific tissue-like structures and functions better than two-dimensional (2D) cultures. 2D cultures are easy to set up, but lack tumor cell–tumor cell, tumor cell–stromal cell, and tumor cell–extracellular matrix interactions of a typical tumor (Kurioka et al 2011). 3D tissue technology may be used to produce tissue models of cancer, which may help glean new information about cancer development and biology by recreating the in vivo tumor phenotype (Hutmacher et al 2010, Jessup et al 1993, Jong Bin 2005, Ingram et al 2010). A cancer model may also be used for high-throughput pre-animal and preclinical evaluation of anticancer drug candidates because 3D tissues can mimic the tissue response and drug resistance better than 2D cultures (Burdett et al 2010, Kunz-Schughart et al 2004). 3D co-cultures may contribute to cancer research when heterogeneous cell populations (cancer along with cancer stem/tumor-initiating cell populations) are used to generate multicellular heterotypic spheroids (Hirschhaeuser et al 2010, Friedrich et al 2007). Cancer stem cells are now considered as adjunct targets that must be shut down to decrease the possibility of tumor relapse after chemotherapy or immunotherapy. Tumor-immune cell co-cultures can be considered as models for testing novel immunotherapeutic treatment strategies. 3-D model tissue constructs may as well, provide in vitro systems to improve the predictive value of cell-based assays in toxicology and food research (Mazzoleni et al 2009)
Model endothelial cells (Sanford et al 2002), skeletal muscle (Marquette et al 2007), erythroid cells (Sytkowski and Davis 2001), adipose tissue (Frye and Patrick 2006), cortical-like tissues (Ma 2008), hepatic tissue (Ishikawa2011), vaginal epithelial cells (Hjelm et al 2009), human intestinal epithelial cells (Skardal et al 2010), cardiac cells (Rungarunlert et al 2011), retina-like structures (Dutt et al 2003) and lacrimal gland acinar cells (Schrader et al 2009) have been constructed under microgravity conditions. 3D models of melanoma (Marrero et al 2009, Licato et al 2001), carcinoma (Nakamura et al 2002), colon carcinoma (Goodwin et al 1992), breast cancer (Vamvakidou et al 2007), lung cancer (Vertrees et al 2009), neuroblastoma (Redden and Doolin 2011), hepatocellular carcinoma (Tang et al 2011) and ovarian and endometrial cancer (Grun et al 2009, Goodwin et al 1997) have been engineered using microgravity bioreactors.
Carvalho et al have expanded the application of microgravity tissue engineering by developing a 3D tissue culture model from human intestinal epithelial HCT-8 cells using RCCS for the study of attach and efface lesion formation by enteropathogenic and enterohemorrhagic Escherichia coli (Carvalho et al 2005). A 3D Huh7 cell culture system was also engineered for the study of hepatitis C virus infection (Sainz et al 2009). Human norovirus infection of Caco-2 cells was modeled by growing tissue in a RWV bioreactor to develop an infectivity assays (Straub et al 2011). Researchers have simulated the HIV pathogenesis in artificial lymphoid tissue (Margolis et al 1997), Borrelia burgdorferi virulence in human tonsillar tissue (Duray et al 2005) and cryptosporidiosis in the HCT-8 (intestinal cells) organoid model (Alcantara Warren et al 2008). Cyclospora parasite has been grown with cells from the small intestine in microgravity bioreactors (Vastag 2001). Applications of RWV bioreactor in establishing organotypic 3D cell culture models to study host–pathogen interactions has been reviewed by Barrila et al (2010).
Current commercial tissue products
Many commercial tissue engineering products are currently available in clinic, however most are not from microgravity origin. The presence of these products demonstrates that tissue engineering is a viable medical and commercial approach.
Carticel®, as the first cell therapy (cartilage) product approved by the FDA, has proved very successful clinically; no reports of serious adverse effects exist. In this approach, autologous chondrocytes are grown in vitro and then grafted into a cartilage defects (Gillogly and Myers 2005). Matrix-induced Autologous Chondrocyte Implantation (MACI), ChondroArt, co.don chondrotransplant, co.don chondrosphere, BioSeed®-C, NOVOCART®, Cartilage Repair System (CaRe S), ArthroMatrix® and ChondroCelect® are all similar commercial products prepared in a similar approach as Carticel® (Samadikuchaksaraei 2010).
Skin replacement therapies are intended for treatment of acute or chronic skin disorders and cosmetic surgeries. Integra, EpicelTM, Biobrane®, Suprathel®, Matriderm® and Transcyte® are examples of products targeted to burn victims (Hentze et al 2007, Dieckmann et al 2010). Dermagraft®, EpiDex®, Epibase, Laserskin, Permacol®, Oasis® and Apligraf®are available for patients with chronic skin ulcers. BioSeed-MTM and MelanoSeedTMare the two products being used in cosmetic surgery (Samadikuchaksaraei 2010). For an excellent and up to date review of the regenerative medicine in dermatology refer to (Dieckmann et al 2010). Tissue engineered bone products include BioSeed-Oral Bone®, co.don osteotransplant® and Osteocel. Hepatocyte preparations have also shown promise as extracorporeal BALs. These systems are now under study as Extracorporeal Liver Assist Device (ELAD), HepatAssist, Bioartificial Liver Support System (BLSS) and Extracorporeal Liver System (MELS). Tissue engineered vascular products, neural products (for treatment of spinal cord injury) and cellular products for the constructive functional remodeling of the heart after a myocardial infarction seem to be achievable targets for regenerative medicine (Badylak and Nerem 2010).
Future outlook
In this review article, we focused on microgravity tissue engineering of cartilage, bone, liver and pancreas as well as 3D models of different organs; however, other tissues such as epidermis, periodontal ligament and arteries have also been constructed in microgravity (Gao et al 2012, Lei et al 2011, Li et al 2009). The culture of whole sensory organs and other high-density structures in rotating bioreactors can provide in vitro models for physiological and pathophysiological investigations (Arnold et al 2010, Hahn 2008). Very recently, a 3D cell biology model of human hepatocellular carcinoma was constructed in vitro by culturing MHCC97H cells on molecular scaffolds within a RWV bioreactor (Tang et al 2011). A modified RCCS bioreactor, Rotary Cell Culture System! (RCCS!), was used to engineer a 3D model of bone matrix for studying osteocytes’ differentiation and bone matrix formation (Mazzolenia et al 2011). Some researchers speculate that microgravity tissue engineering will allow for testing chemotherapeutics on cells taken from an individual patient and grown in vitro.
However, there are yet some obstacles to overcome after achieving tissue constructs of desired sizes and qualities. One of these issues is the variability of patient response regarding resorption, recellularisation and regeneration of the implanted tissue (Korossis et al 2005). Spontaneous vascularisation of the in vitro grown tissue also remains an issue (Korossis et al 2005). One question that remains to be answered is whether the differences in cell physiology and gene expression in cells and tissues constructed under microgravity could adversely affect patients treated with these products. Bone loss in space for example, has been attributed to changes in gene expression in osteoclasts (Sambandam 2010, Tamma et al 2009). With advances in the field and overcoming these obstacles in near future, we may witness a golden era in which tissue replacement therapy of defective organs will be a viable option.
Ethical Issues
None to be declared.
Conflicts of interest
The authors declare no conflict of interests.
Acknowledgments
Authors are grateful to Sara Saei for her contributions in editing the content of the paper.
References
- Alcantara Warren C, Destura RV, Sevilleja JE, Barroso LF, Carvalho H, Barrett LJ, et al. 2008 Detection of epithelial-cell injury, and quantification of infection, in the HCT-8 organoid model of cryptosporidiosis. J Infect Dis, 198(1), 143-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman G, Horan R, Martin I, Farhadi J, Stark P, Volloch V, et al. 2002 Cell Differentiation by Mechanical Stress. FASEB J, 16(2), 270-272 [DOI] [PubMed] [Google Scholar]
- Araujo JV, Cunha-Reis C, Rada T, Alcantara Warrenda Silva MA, Gomes ME, Yang Y, et al. 2010 Dynamic culture of osteogenic cells in biomimetically coated poly(caprolactone) nanofibre mesh constructs. Tissue Eng Part A, 16(2), 557-563 [DOI] [PubMed] [Google Scholar]
- Arnold HJ, Muller M, Waldhaus J, Hahn H and Lowenheim H . 2010 A Novel Buoyancy Technique Optimizes Simulated Microgravity Conditions for Whole Sensory Organ Culture in Rotating Bioreactors. Tissue Eng Part C Methods, 16(1), 51-61 [DOI] [PubMed] [Google Scholar]
- Ayyaswamy PS and Mukundakrishnan K . 2007 Optimal conditions for simulating microgravity employing NASA designed rotating wall vessels. Acta Astronautica, 60(4-7), 397-405 [Google Scholar]
- Badylak SF and Nerem RM . 2010 Progress in Tissue Engineering and Regenerative Medicine. Proc Natl Acad Sci U S A, 107(8), 3285-3286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrila J, Radtke AL, Crabbe A, Sarker ShF, Herbst-Kralovetz MM, Ott CM, et al. 2010 Organotypic 3D cell culture models: using the rotating wall vessel to study host–pathogen interactions. Nature Rev Microbiol, 8, 791-801 [DOI] [PubMed] [Google Scholar]
- Botchwey EA, Pollack SR, Levine EM and Laurencin CT . 2001 Bone Tissue Engineering in a Rotating Bioreactor Using a Microcarrier Matrix System. J Biomed Mater Res, 55(2), 242-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LA, Arterburn LM, Miller AP, Cowger NL, Hartley SM, Andrews ANNE, et al. 2003 Maintenance of liver functions in rat hepatocytes cultured as spheroids in a rotating wall vessel. In Vitro Cell Dev Biol Anim, 39(1 & 2), 13-20 [DOI] [PubMed] [Google Scholar]
- Burdett E, Kasper FK, Mikos AG and Ludwig JA . 2010 Engineering Tumors: a Tissue Engineering Perspective in Cancer Biology. Tissue Eng Part B: Reviews, 16(3), 351-359 [DOI] [PubMed] [Google Scholar]
- Cameron DF, Hushen JJ and Nazian SJ . 2001. a Formation of Insulin-Secreting, Sertoli-Enriched Tissue Constructs by Microgravity Coculture of Isolated Pig Islets and Rat Sertoli Cells. In Vitro Cell Dev Biol Anim, 37(8), 490-498 [DOI] [PubMed] [Google Scholar]
- Cameron DF, Hushen JJ, Nazian SJ, Willing ALIS, Saporta SAM and Sanberg PR . 2001. b Formation of Sertoli Cell-Enriched Tissue Constructs Utilizing Simulated Microgravity Technology. Ann N Y Acad Sci, 944(1), 420-428 [DOI] [PubMed] [Google Scholar]
- Carvalho HM, Teel LD, Goping G and O'Brien AD . 2005 A Three-Dimensional Tissue Culture Model for the Study of Attach and Efface Lesion Formation by Enteropathogenic and Enterohaemorrhagic Escherichia Coli. Cell Microbiol, 7(12), 1771-1781 [DOI] [PubMed] [Google Scholar]
- Carver SE and Heath CA . 1999 Semi-Continuous Perfusion System for Delivering Intermittent Physiological Pressure to Regenerating Cartilage. Tissue eng, 5(1), 1-11 [DOI] [PubMed] [Google Scholar]
- Dabos KJ, Nelson LJ, Bradnock TJ, Parkinson JA, Sadler IH, Hayes PC, et al. 2001 The Simulated Microgravity Environment Maintains Key Metabolic Functions and Promotes Aggregation of Primary Porcine Hepatocytes. Biochim Biophys Acta - General Subjects, 1526(2), 119-130 [DOI] [PubMed] [Google Scholar]
- Dieckmann C, Renner R, Milkova L and Simon JC . 2010 Regenerative Medicine in Dermatology: Biomaterials, Tissue Engineering, Stem Cells, Gene Transfer and Beyond. Exp dermatol, 19(8), 697-706 [DOI] [PubMed] [Google Scholar]
- Dintenfass L . 1986 Execution of "ARC" Experiment on Space Shuttle" Discovery" STS 51-C: Some Results on Aggregation of Red Blood Cells Under Zero Gravity. Biorheology, 23(4), 331-347 [DOI] [PubMed] [Google Scholar]
- Duke J, Daane E, Arizpe J and Montufar-Solis D . 1996 Chondrogenesis in Aggregates of Embryonic Limb Cells Grown in a Rotating Wall Vessel. Adv Space Res, 17(6-7), 289-293 [DOI] [PubMed] [Google Scholar]
- Duray PH, Yin SR, Ito Y, Bezrukov L, Cox C, Cho MS, Fitzgerald W, et al. 2005 Invasion of human tissue ex vivo by Borrelia burgdorferi. J Infect Dis, 191(10), 1747-1754 [DOI] [PubMed] [Google Scholar]
- Dutt K, Harris-Hooker S, Ellerson D, Layne D, Kumar R and Hunt R . 2003 Generation of 3D Retina-Like Structures From a Human Retinal Cell Line in a NASA Bioreactor. Cell Transplantation, 12(7), 717-731 [DOI] [PubMed] [Google Scholar]
- EL-Haj AJ and Cartmell SH. 2010. Bioreactors for bone tissue engineering. Proc IMechE, Part H: J Eng Med, 224, 1523-1532. [DOI] [PubMed]
- Emin N, Koc A, Durkut S, Elcin AE and Elcin YM . 2008 Engineering of Rat Articular Cartilage on Porous Sponges: Effects of TGF-B 1 and Microgravity Bioreactor Culture. Artif Cells, Blood Substitutes and Biotechnology, 123-137 [DOI] [PubMed] [Google Scholar]
- Facer SR, Zaharias RS, Andracki ME, Lafoon J, Hunter SK and Schneider GB . 2005 Rotary Culture Enhances Pre-osteoblast Aggregation and Mineralization. J Dent Res, 84(6), 542-547 [DOI] [PubMed] [Google Scholar]
- Farrag SI . 2009 Determination of microcarrier radius that can be cultured in a rotational wall vessel bioreactor. Egyptian Journal of Medical Human Genetics, 10(2), 208-217 [Google Scholar]
- Francis KM, O'Connor KC and Spaulding GF . 1997 Cultivation of Fall Armyworm Ovary Cells in Simulated Microgravity. In Vitro Cell Dev Biol Anim, 33(5), 332-336 [DOI] [PubMed] [Google Scholar]
- Freed LE, Hollander AP, Martin I, Barry JR, Langer R and Vunjak-Novakovic G . 1998 Chondrogenesis in a Cell-Polymer-Bioreactor System. Exp Cell Res, 240(1), 58-65 [DOI] [PubMed] [Google Scholar]
- Freed LE, Langer R, Martin I, Pellis NR and Vunjak-Novakovic G . 1997. a Tissue Engineering of Cartilage in Space. Proc Natl Acad Sci U S A, 94(25), 13885-13890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed LE and Vunjak-Novakovic G . 1997. b Microgravity Tissue Engineering. In Vitro Cell Dev Biol Anim, 33(5), 381-385 [DOI] [PubMed] [Google Scholar]
- Freed LE and Vunjak-Novakovic G . 1995 Cultivation of Cell-Polymer Tissue Constructs in Simulated Microgravity. Biotechnol bioeng, 46(4), 306-313 [DOI] [PubMed] [Google Scholar]
- Friedrich J, Ebner R and Kunz-Schughart LA . 2007 Experimental Anti-Tumor Therapy in 3-D: Spheroids--old Hat or New Challenge? Int J Radiat Biol, 83(11-12), 849-871 [DOI] [PubMed] [Google Scholar]
- Frye CA and Patrick CW . 2006 Three-dimensional adipose tissue model using low shear bioreactors. In Vitro Cell Dev Biol Anim, 42(5-6), 109-114 [DOI] [PubMed] [Google Scholar]
- Gao F, Cheng JH, Xue JH, Bai YG, Chen MS, Huang WQ, et al. 2012 In-Vivo and Ex-Vivo Studies on Region-Specific Remodeling of Large Elastic Arteries Due to Simulated Weightlessness and Its Prevention by Gravity-Based Countermeasure. Sheng Li Xue Bao, 64(1), 14-26 [PubMed] [Google Scholar]
- Ghaffari T, Nouri M, Saei AA and Rashidi MR. 2012. Aldehyde and Xanthine Oxidase Activities in Tissues of Streptozotocin-Induced Diabetic Rats: Effects of Vitamin E and Selenium Supplementation. Biol Trace Elem Res, [Epub ahead of print]. [DOI] [PubMed]
- Gillogly SD and Myers TH . 2005 Treatment of Full-Thickness Chondral Defects With Autologous Chondrocyte Implantation. Orthop Clin North Am, 36(4), 433-446 [DOI] [PubMed] [Google Scholar]
- Goldstein AS, Juarez TM, Helmke CD, Gustin MC and Mikos AG . 2001 Effect of Convection on Osteoblastic Cell Growth and Function in Biodegradable Polymer Foam Scaffolds. Biomaterials, 22(11), 1279-1288 [DOI] [PubMed] [Google Scholar]
- Goodwin TJ, Prewett TL, Spaulding GF and Becker JL . 1997 Three-Dimensional Culture of a Mixed Mullerian Tumor of the Ovary: Expression of in vivo Characteristics. In Vitro Cell Dev Biol, 33(5), 366-374 [DOI] [PubMed] [Google Scholar]
- Goodwin TJ, Milburn Jessup and Wolf DA . 1992 Morphologic Differentiation of Colon Carcinoma Cell Lines HT-29 and HT-29KM in Rotating-Wall Vessels. In Vitro Cell Dev Biol, 28(1), 47-60 [DOI] [PubMed] [Google Scholar]
- Granet C, Laroche N, Vico L, Alexandre C and Lafage-Proust MH . 1998 Rotating-Wall Vessels, Promising Bioreactors for Osteoblastic Cell Culture: Comparison With Other 3D Conditions. Med Biol Eng Comput, 36(4), 513-519 [DOI] [PubMed] [Google Scholar]
- Grun B, Benjamin E, Sinclair J, Timms JF, Jacobs IJ, Gayther SA, et al. 2009 Three-dimensional in vitro cell biology models of ovarian and endometrial cancer. Cell prolif, 42(2), 219-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H, Muller M and Lowenheim H . 2008 Whole organ culture of the postnatal sensory inner ear in simulated microgravity. J Neurosci Methods, 171(1), 60-71 [DOI] [PubMed] [Google Scholar]
- Hammond TG and Hammond JM . 2001 Optimized Suspension Culture: the Rotating-Wall Vessel. Am J Physiol Renal Physiol, 281(1), F12-F25 [DOI] [PubMed] [Google Scholar]
- Han X, Qiu L, Zhang Y, Kong Q, Wang H, Wang H, et al. 2009 Transplantation of Sertoli-Islet Cell Aggregates Formed by Microgravity: Prolonged Survival in Diabetic Rats. Exp Biol Med (Maywood), 234(5), 595-603 [DOI] [PubMed] [Google Scholar]
- Hentze H, Graichen R and Colman A . 2007 Cell Therapy and the Safety of Embryonic Stem Cell-Derived Grafts. Trends Biotechnol, 25(1), 24-32 [DOI] [PubMed] [Google Scholar]
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W and Kunz-Schughart LA . 2010 Multicellular Tumor Spheroids: An Underestimated Tool Is Catching Up Again. J biotechnol, 148(1), 3-15 [DOI] [PubMed] [Google Scholar]
- Hjelm BE, Berta AN, Nickerson ChA, Arntzen ChJ and Herbst-Kralovetz MM . 2010 Development and Characterization of a Three-Dimensional Organotypic Human Vaginal Epithelial Cell Model. Biol Reprod, 82(3), 617-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochleitner B, Hengster P, Bucher H, Ladurner R, Schneeberger S, Krismer A, et al. 2006 Significant Survival Prolongation in Pigs With Fulminant Hepatic Failure Treated With a Novel Microgravity-Based Bioartificial Liver. Artif organs, 30(12), 906-914 [DOI] [PubMed] [Google Scholar]
- Hochleitner B, Hengster P, Duo L, Bucher H, Klima G and Margreiter R . 2005 A Novel Bioartificial Liver With Culture of Porcine Hepatocyte Aggregates Under Simulated Microgravity. Artif organs, 29(1), 58-66 [DOI] [PubMed] [Google Scholar]
- Hoffman RM . 1993 To do tissue culture in two or three dimension? That is the question. Stem Cells, 11, 105-111 [DOI] [PubMed] [Google Scholar]
- Hou Y, Song C, Xie WJ, Wei Z, Huang RP, Liu W, et al. 2009 Excellent Effect of Three-Dimensional Culture Condition on Pancreatic Islets. Diabetes Res Clin Pract, 86(1), 11-15 [DOI] [PubMed] [Google Scholar]
- Hu JC and Athanasiou KA . 2005 Low-Density Cultures of Bovine Chondrocytes: Effects of Scaffold Material and Culture System. Biomaterials, 26(14), 2001-2012 [DOI] [PubMed] [Google Scholar]
- Hutmacher DW, Loessner D, Rizzi S, Kaplan DL, Mooney DJ and Clements JA . 2010 Can Tissue Engineering Concepts Advance Tumor Biology Research? Trends Biotechnol, 28(3), 125-133 [DOI] [PubMed] [Google Scholar]
- Hwang YS, Cho J, Tay F, Heng JYY, Ho R, Kazarian SG, et al. 2009 The Use of Murine Embryonic Stem Cells, Alginate Encapsulation, and Rotary Microgravity Bioreactor in Bone Tissue Engineering. Biomaterials, 30(4), 499-507 [DOI] [PubMed] [Google Scholar]
- Hymer WC, Grindeland RE, Salada T, Cenci R, Krishnan K, Mukai C, et al. 1996 Feeding Frequency Affects Cultured Rat Pituitary Cells in Low Gravity. J biotechnol, 47(2-3), 289-312 [DOI] [PubMed] [Google Scholar]
- Ingram M, Techy GB, Ward BR, Imam SA, Atkinson R, Ho H, et al. 2010 Tissue engineered tumor models. Biotech Histochem, 85(4), 213-229 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Sekine K, Okamura A, Zheng Y, Ueno Y and Koike N . 2011 Reconstitution of hepatic tissue architectures from fetal liver cells obtained from a three-dimensional culture with a rotating wall vessel bioreactor. J Biosci Bioeng, 111(6), 711-718 [DOI] [PubMed] [Google Scholar]
- Jessup JM, Goodwin TJ and Spaulding G . 1993 Prospects for Use of Microgravity-Based Bioreactors to Study Three-Dimensional Host-Tumor Interactions in Human Neoplasia. J Cell Biochem, 51(3), 290-300 [DOI] [PubMed] [Google Scholar]
- Jin F, Zhang Y, Xuan K, He D, Deng T, Tang L, et al. 2010 Establishment of Three-Dimensional Tissue Engineered Bone Constructs Under Microgravity-Simulated Conditions. Artif organs, 34(2), 118-125 [DOI] [PubMed] [Google Scholar]
- Alcantara Warrenda SilvaJong Bin K . 2005 Three-Dimensional Tissue Culture Models in Cancer Biology. Semin Cancer Biol, 15(5), 365-377 [DOI] [PubMed] [Google Scholar]
- Khaoustov VI, Darlington GJ, Soriano HE, Krishnan B, Risin D, Pellis NR, et al. 1999 Induction of Three-Dimensional Assembly of Human Liver Cells by Simulated Microgravity. In Vitro Cell Dev Biol Anim, 35(9), 501-509 [DOI] [PubMed] [Google Scholar]
- Koc A, Emin N, Elcin AE and Elcin YM . 2008 In Vitro Osteogenic Differentiation of Rat Mesenchymal Stem Cells in a Microgravity Bioreactor. Journal of Bioactive and Compatible Polymers, 23(3), 244-261 [Google Scholar]
- Korossis S, Bolland F, Kearney J, Fisher J and Ingham E . 2005 Bioreactors in Tissue Engineering. Topics Tissue Eng, 2, 1-23 [Google Scholar]
- Kunz-Schughart LA, Freyer JP, Hofstaedter F and Ebner R . 2004 The Use of 3-D Cultures for High-Throughput Screening: the Multicellular Spheroid Model. J Biomol Screen, 9(4), 273-285 [DOI] [PubMed] [Google Scholar]
- Kurioka D, Takagi A, Yoneda M, Hirokawa Y, Shiraishi T and Watanabe M . 2011 Multicellular Spheroid Culture Models: Applications in Prostate Cancer Research and Therapeutics. J Cancer Sci Ther, 3(3), 060-065 [Google Scholar]
- Kwon O, Devarakonda SB, Sankovic JM and Banerjee RK . 2008 Oxygen transport and consumption by suspended cells in microgravity: a multiphase analysis. Biotechnol Bioeng, 99(1), 99-107 [DOI] [PubMed] [Google Scholar]
- Kyriakidou K, Lucarini G, Zizzi A, Salvolini E, Alcantara Warrenda SilvaJong BinMattioli Belmonte M, Mollica F, et al. 2008 Dynamic Co-Seeding of Osteoblast and Endothelial Cells on 3D Polycaprolactone Scaffolds for Enhanced Bone Tissue Engineering. Journal of Bioactive and Compatible Polymers, 23(3), 227-243 [Google Scholar]
- Langer R . 1997 Tissue Engineering: a New Field and Its Challenges. Pharm Res, 14(7), 840-841 [DOI] [PubMed] [Google Scholar]
- Lei XH, Ning LN, Cao YJ, Liu S, Zhang SB, Qiu ZF, et al. 2011 NASA-Approved Rotary Bioreactor Enhances Proliferation of Human Epidermal Stem Cells and Supports Formation of 3D Epidermis-Like Structure. PLoS One, 6(11), e26603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ma Z, Niu Z, Qian H, Xuan D, Hou R, et al. 2009 NASA-Approved Rotary Bioreactor Enhances Proliferation and Osteogenesis of Human Periodontal Ligament Stem Cells. Stem Cells Dev, 18(9), 1273-1282 [DOI] [PubMed] [Google Scholar]
- Licato LL, Prieto VG and Grimm EA . 2001 A novel preclinical model of human malignant melanoma utilizing bioreactor rotating-wall vessels. In Vitro Cell Dev Biol Anim, 73(3), 121-126 [DOI] [PubMed] [Google Scholar]
- Lv Q, Nair L and Laurencin CT . 2008 Fabrication, characterization, and in vitro evaluation of poly(lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors . J Biomed Mater Res A, 91A(3), 679-691 [DOI] [PubMed] [Google Scholar]
- Ma W, Tavakoli T, Chen S, Maric D, Liu JL, O'Shaughnessy TJ, et al. 2008 Reconstruction of Functional Cortical-like Tissues from Neural Stem and Progenitor Cells. Tissue Eng Part A, 14(10), 1673-1686 [DOI] [PubMed] [Google Scholar]
- Margolis LB, Fitzgerald W, Glushakova S, Hatfill S, Amichay N, Baibakov B, et al. 1997 Lymphocyte trafficking and HIV infection of human lymphoid tissue in a rotating wall vessel bioreactor. AIDS Res Hum Retroviruses, 13(16), 1411-1420 [DOI] [PubMed] [Google Scholar]
- Marquette M, Byerly D and Sognier M . 2007 A Novel in Vitro Three-Dimensional Skeletal Muscle Model. In Vitro Cell Dev Biol Anim, 43(7), 255-263 [DOI] [PubMed] [Google Scholar]
- Marrero B, Messina JL and Heller R . 2009 Generation of a Tumor Spheroid in a Microgravity Environment As a 3D Model of Melanoma. In Vitro Cell Dev Biol Anim, 9(45), 523-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Y and Vermette P . 2005 Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials, 26(35), 7481-7503 [DOI] [PubMed] [Google Scholar]
- Mazzoleni G, Boukhechba F, Steimberg N, Boniotti J, Bouler JM and Rochet N . 2011 Impact of Dynamic Culture in the RCCS! Bioreactor on a Three-Dimensional Model of Bone Matrix Formation. Procedia Engineering, 10, 3662-3667 [Google Scholar]
- Mazzoleni G, Di Lorenzo and Steimberg N . 2009 Modelling Tissues in 3D: the Next Future of Pharmaco-Toxicology and Food Research? Genes Nutr, 4(1), 13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi N, Khandanic A, Camirand A and Harrison RE . 2011 Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone, 49(5), 965-974 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kuga H, Morisaki T, Baba E, Sato N, Mizumoto K, et al. 2002 Simulated Microgravity Culture System for a 3-D Carcinoma Tissue Model. Biotechniques, 33, 1068-1076 [DOI] [PubMed] [Google Scholar]
- Nelson LJ, Walker SW, Hayes PC and Plevris JN . 2010 Low-Shear Modelled Microgravity Environment Maintains Morphology and Differentiated Functionality of Primary Porcine Hepatocyte Cultures. Cells Tissues Organs, 192, 125-140 [DOI] [PubMed] [Google Scholar]
- Nettles DL, Elder SH and Gilbert JA . 2002 Potential Use of Chitosan As a Cell Scaffold Material for Cartilage Tissue Engineering. Tissue Eng, 8(6), 1009-1016 [DOI] [PubMed] [Google Scholar]
- Ohyabu Y, Tanaka J, Ikada Y and Uemura T . 2009 Cartilage Tissue Regeneration From Bone Marrow Cells by RWV Bioreactor Using Collagen Sponge Scaffold. Materials Science and Engineering: C, 29(4), 1150-1155 [Google Scholar]
- Ohyabu Y, Kida N, Kojima H, Taguchi T, Tanaka J and Uemura T . 2006 Cartilaginous Tissue Formation From Bone Marrow Cells Using Rotating Wall Vessel (RWV) Bioreactor. Biotechnol bioeng, 95(5), 1003-1008 [DOI] [PubMed] [Google Scholar]
- Partap S, Plunkett NA and O'Brien FJ . 2010 Bioreactors in Tissue Engineering. Tissue Engineering (Lazinica A, ed), 323-336 [Google Scholar]
- Qiu QQ, Ducheyne P and Ayyaswamy PS . 2001 3D Bone Tissue Engineered With Bioactive Microspheres in Simulated Microgravity. In Vitro Cell Dev Biol Anim, 37(3), 157-165 [DOI] [PubMed] [Google Scholar]
- Qiu QQ, Ducheyne P and Ayyaswamy PS . 1999 Fabrication, Characterization and Evaluation of Bioceramic Hollow Microspheres Used As Microcarriers for 3-D Bone Tissue Formation in Rotating Bioreactors. Biomaterials, 20(11), 989-1001 [DOI] [PubMed] [Google Scholar]
- Redden RA and Doolin EJ . 2011 Microgravity assay of neuroblastoma: in vitro aggregation kinetics and organoid morphology correlate with MYCN expression. In Vitro Cell Dev Biol Anim, 47(4), 312-317 [DOI] [PubMed] [Google Scholar]
- Rucci N, Rufo A, Alamanou M and Teti A . 2007 Modeled Microgravity Stimulates Osteoclastogenesis and Bone Resorption by Increasing Osteoblast RANKL/OPG Ratio. J cell biochem, 100(2), 464-473 [DOI] [PubMed] [Google Scholar]
- Rungarunlert S, Klincumhom N, Bock I, Nemes C, Techakumphu M, Pirity MK, et al. 2011 Enhanced cardiac differentiation of mouse embryonic stem cells by use of the slow-turning, lateral vessel (STLV) bioreactor. Biotechnol Lett, 33(8), 1565-1573 [DOI] [PubMed] [Google Scholar]
- Rutzky LP, Bilinski S, Kloc M, Phan T, Zhang H, Katz SM, et al. 2002 Microgravity Culture Condition Reduces Immunogenicity and Improves Function of Pancreatic Islets1. Transplantation, 74(1), 13-21 [DOI] [PubMed] [Google Scholar]
- Ryan EA, Lakey JR, Paty BW, Imes S, Korbutt GS, Kneteman NM, et al. 2002 Successful Islet Transplantation: Continued Insulin Reserve Provides Long-Term Glycemic Control. Diabetes, 51(7), 2148-2157 [DOI] [PubMed] [Google Scholar]
- Sainz B, TenCate V and Uprichard SL . 2009 Three-dimensional Huh7 cell culture system for the study of Hepatitis C virus infection. Virol Journal, 6:103, 6:103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Mishima H, Ishii T, Akaogi H, Yoshioka T and Ohyabu Y . 2009 Rotating three-dimensional dynamic culture of adult human bone marrow-derived cells for tissue engineering of hyaline cartilage. J Orthop Res, 27(4), 517-521 [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A . 2007 Scientific and Industrial Status of Tissue Engineering. Afr J Biotechnol, 6(25), 2897-2909 [Google Scholar]
- Sambandam Y, Blanchard JJ, Daughtridge G, Kolb RJ, Shanmugarajan S, Pandruvada SN, et al. 2010 Microarray profile of gene expression during osteoclast differentiation in modelled microgravity. J Cell Biochem, 111, 1179-1187 [DOI] [PubMed] [Google Scholar]
- Sanford GL, Ellerson D, Melhado-Gardner C, Sroufe AE and Harris-Hooker S . 2002 Three-Dimensional Growth of Endothelial Cells in the Microgravity-Based Rotating Wall Vessel Bioreactor. In Vitro Cell Dev Biol Anim, 38(9), 493-504 [DOI] [PubMed] [Google Scholar]
- Schneider GB, Boehrs JK, Hoopes JV and Seabold DA . 2011 Use of 3-dimensional environments to engineer osseous-like tissue. Journal of Developmental Biology and Tissue Engineering, 3(4), 42-47 [Google Scholar]
- Schrader S, Kremling C, Klinger M, Laqua H and Geerling G . 2009 Cultivation of lacrimal gland acinar cells in a microgravity environment. Br J Ophthalmol, 93(8), 1121-1125 [DOI] [PubMed] [Google Scholar]
- Schwarz RP, Goodwin TJ and Wolf DA . 1992 Cell Culture for Three-Dimensional Modeling in Rotating-Wall Vessels: an Application of Simulated Microgravity. J Tissue Cult Methods, 14(2), 51-57 [DOI] [PubMed] [Google Scholar]
- Shapiro AMJ and Lakey JRT . 2000. a Future Trends in Islet Cell Transplantation. Diabetes Technol Ther, 2(3), 449-452 [DOI] [PubMed] [Google Scholar]
- Shapiro AMJ, Lakey JRT, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. 2000. b Islet Transplantation in Seven Patients With Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N Engl J Med, 343(4), 230-238 [DOI] [PubMed] [Google Scholar]
- Skardal A, Sarker SF, Crabbé A, Nickerson CA and Prestwich GD . 2010 The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. Biomaterials, 31(32), 8426-8435 [DOI] [PubMed] [Google Scholar]
- Song K, Liu T, Cui Z, Li X and Ma X . 2008 Three-Dimensional Fabrication of Engineered Bone With Human Bio-Derived Bone Scaffolds in a Rotating Wall Vessel Bioreactor. J Biomed Mater Res A, 86A(2), 323-332 [DOI] [PubMed] [Google Scholar]
- Song K, Yang Z, Liu T, Zhi W, Li X, Deng L, et al. 2006 Fabrication and Detection of Tissue-Engineered Bones With Bio-Derived Scaffolds in a Rotating Bioreactor. Biotechnol Appl Biochem, 45(2), 65-74 [DOI] [PubMed] [Google Scholar]
- Song C, Duan XQ, Li X, Han LO, Xu P, Song CF, et al. 2004. a Experimental Study of Rat Beta Islet Cells Cultured Under Simulated Microgravity Conditions. Acta Biochim Biophys Sin (Shanghai), 36(1), 47-50 [DOI] [PubMed] [Google Scholar]
- Song C, Duan XQ, Zhou Y, Li X, Han LO, Xu P, et al. 2004. b [Experimental Study on Islet Cells in Rats Under Condition of Three-Dimensional Microgravity]. Zhonghua Wai Ke Za Zhi, 42(9), 559-561 [PubMed] [Google Scholar]
- Stamenkovic V, Keller G, Nesic D, Cogoli A and Grogan SP . 2010 Neocartilage Formation in 1 G, Simulated, and Microgravity Environments: Implications for Tissue Engineering. Tissue Eng Part A, 16(5), 1729-1736 [DOI] [PubMed] [Google Scholar]
- Straub TM, Bartholomew RA, Valdez CO, Valentine NB, Dohnalkova A, Ozanich RM, et al. 2011 Human Norovirus Infection of Caco-2 Cells Grown as a 3-Dimensional Tissue Structure. J Water Health, 9(2), 225-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RM, Sordat B, Bamat J, Gabbert H, Bourrat B and Mueller-Klieser W . 1986 Oxygenation and Differentiation in Multicellular Spheroids of Human Colon Carcinoma. Cancer res, 46(10), 5320-5329 [PubMed] [Google Scholar]
- Sytkowski AJ and Davis KL . 2001 Erythroid Cell Growth and Differentiation in vitro in the Simulated Microgravity Environment of the NASA Rotating Wall Vessel Bioreactor. In Vitro Cell Dev Biol Anim, 37(2), 79-83 [DOI] [PubMed] [Google Scholar]
- Tamma R, Colaianni G, Camerino C, Alcantara Warrenda SilvaJong BinMattioli BelmonteDi Benedetto A, Greco G and Strippoli M . 2009 Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J, 23(8), 23(8) 2549-2554 [DOI] [PubMed] [Google Scholar]
- Tang J, Cui J, Chen R, Guo K, Kang X, Li Y, Gao D, et al. 2011 A three-dimensional cell biology model of human hepatocellular carcinoma in vitro. Tumor Biol, 32(3), 469-479 [DOI] [PubMed] [Google Scholar]
- Thiel K, Schenk M, Etspuler A, Schenk T, Morgalla MH, Konigsrainer A, et al. 2011 A Simple Dummy Liver Assist Device Prolongs Anhepatic Survival in a Porcine Model of Total Hepatectomy by Slight Hypothermia. BMC Gastroenterol, 11, 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SC and Mandel TE . 1990 Fetal Pig Pancreas Preparation and Assessment of Tissue for Transplantation, and Its in vivo Development and Function in Athymic (Nude) Mice. Transplantation, 49(3), 571-581 [DOI] [PubMed] [Google Scholar]
- Turhani D, Watzinger E, Weissenbock M, Cvikl B, Thurnher D, Wittwer G, et al. 2005 Analysis of Cell-Seeded 3-Dimensional Bone Constructs Manufactured in vitro With Hydroxyapatite Granules Obtained From Red Algae. J Oral Maxillofac Surg, 63(5), 673-681 [DOI] [PubMed] [Google Scholar]
- Unsworth BR and Lelkes PI. 2000. Growing Tissues in Microgravity, In: Principles of Tissue Engineering, Lanza R, Langer R, Vacant J, Eds. Academic Press, San Diego, pp 157-164.
- Unsworth BR and Lelkes PI . 1998 Growing Tissues in Microgravity. Nat med, 4(8), 901-907 [DOI] [PubMed] [Google Scholar]
- Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A and Langer R . 1988 Selective Cell Transplantation Using Bioabsorbable Artificial Polymers As Matrices. J pediatr surg, 23(1), 3-9 [DOI] [PubMed] [Google Scholar]
- Vamvakidou AP, Mondrinos MJ, Petushi SP, Garcia FU, Lelkes PI and Tozeren A . 2007 Heterogeneous Breast Tumoroids: An in vitro Assay for Investigating Cellular Heterogeneity and Drug Delivery. J Biomol Screen, 12(1), 13-20 [DOI] [PubMed] [Google Scholar]
- Vastag B . 2001 Cell Biology Update: A Decade of Simulating Space on Earth. JAMA, 285(17), 2181-2182 [DOI] [PubMed] [Google Scholar]
- Vertrees RA, McCarthy M, Solley T, Popov VL, Roaten J, Pauley M, et al. 2009 Development of a three-dimensional model of lung cancer using cultured transformed lung cells. Cancer Biol Ther, 8(4), 356-365 [DOI] [PubMed] [Google Scholar]
- Wu FJ, Peshwa MV, Cerra FB and Hu WS . 1995 Entrapment of Hepatocyte Spheroids in a Hollow Fiber Bioreactor As a Potential Bioartificial Liver. Tissue eng, 1(1), 29-40 [DOI] [PubMed] [Google Scholar]
- Wurm M, Lubei V, Caronna M, Hermann M, Buttiglieri S, Bodamer O, et al. 2009 Introduction of a Novel Prototype Bioartificial Liver Support System Utilizing Small Human Hepatocytes in Rotary Culture. Tissue Eng Part A, 15(5), 1063-1073 [DOI] [PubMed] [Google Scholar]
- Yeatts AB and Fisher JB . 2011 Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone, 48, 171-181 [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Mishima H, Ohyabu Y, Sakai S, Akaogi H, Ishii T, et al. 2007 Repair of Large Osteochondral Defects With Allogeneic Cartilaginous Aggregates Formed From Bone Marrow-Derived Cells Using RWV Bioreactor. J orthop res, 25(10), 1291-1298 [DOI] [PubMed] [Google Scholar]
- Yu X, Botchwey EA, Levine EM, Pollack SR and Laurencin CT . 2004 Bioreactor-Based Bone Tissue Engineering: the Influence of Dynamic Flow on Osteoblast Phenotypic Expression and Matrix Mineralization . Proc Natl Acad Sci U S A, 101(31), 11203-11208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuge L, Kajiume T, Tahara H, Kawahara Y, Umeda C and Yoshimoto R . 2006 Microgravity potentiates stem cell proliferation while sustaining the capability of differentiation. Stem Cells Dev, 15(6), 921-929 [DOI] [PubMed] [Google Scholar]


