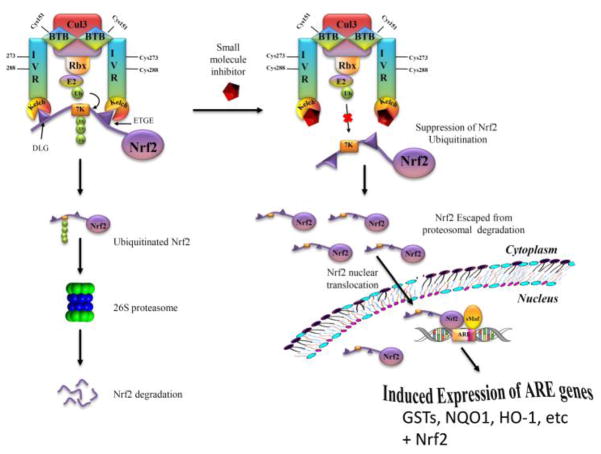
Fig. 1. A model depicting Nrf2 activation by a direct inhibitor of Keap1-Nrf2 interaction.
Nrf2 is sequestered in the cytosol by its protein inhibitor, Keap1, and is transcriptionally inactive. A direct inhibitor binds to the Kelch domain of Keap1 and suppresses the ubiquitination of Nrf2, which decreases degradation of Nrf2 and allows more Nrf2 to translocate to the nucleus and form a transcriptionally active complex with Maf, leading to the induced expression of Nrf2 itself and oxidative stress response enzymes that deactivate reactive oxygen species and electrophiles.