Abstract
To evaluate the feasibility of an iPad-based documented patient briefing for Magnetic Resonance Imaging (MRI) examinations. A standard briefing sheet and questionnaire for a MRI scan was converted from paper form into an iPad application. Twenty patients, who had been referred for an MRI scan, were briefed about the examination in paper form as well as via the iPad application before performing the MRI scan. Time each patient needed for the briefing and the number of questions that came up were documented. Patients’ acceptance of the electronic briefing was assessed using a questionnaire. The mean processing time was 2.36 min (range 0.58 to 09.35 min., standard deviation ±2.05 min) for the paper-based briefing and 4.15 min (range 1.56 to 13.48 min, SD ± 2.36 min) for the app-based briefing. Concerning technical aspects, patients asked two questions during the app-based briefing; no questions arose during the paper-based briefing. Six patients preferred electronic briefing and four patients, the paper-based form. No patient preferred the electronic form with additional multimedial information. Eight participants did not mind which briefing version was used; two participants did not express their preference at all. Our experiences showed that electronic briefing using an iPad is feasible and has the potential to become a user-friendly alternative to the conventional paper-based approach. Owing to the broad range of the results, a follow-up study will seek to determine the influencing factors on processing time and other potential questions.
Keywords: iPad, Electronic patient consent, Tablet PC, App, Feasibility, Apple
Introduction
Smart phones and modern tablet computers are becoming increasingly popular [1]. Especially the introduction of the iPad® by Apple (Cupertino, California, USA) on September 27, 2010 was a decisive moment in the development of this technology, but also other manufacturers and operating systems, such as Google’s Android (Mountain View, California, USA), were also able to gain a foothold on the market. Customers appreciate their universal employability as well as their uncomplicated and intuitive interface with direct on-screen interaction. It is possible to expand a tablet’s software with the help of additional applications (also known as “apps”), which are programs that can be purchased at online software markets, such as Apple’s AppStore® or Google’s PlayStore®. The number of available apps has increased rapidly in the last couple of years. On August 1, 2012, more than 4,680 programs were listed under the category “medicine.” Theoretically, any programmer can create and sell a program via such an online platform. Depending on the concept of the platform owner, the new software is tested for viruses and the content sometimes also for its suitability. However, an external validation process of these applications in terms of their medical correctness has not been conducted so far, which prompted the US Food and Drug Administration to tackle the problem in order to ensure the safety of patients [2, 3]. According to the agency, such applications constitute a medical product and require a corresponding authorization process [4]. Results of preliminary studies dealing with the use of tablet computers such as the iPad suggest their feasibility in image reading as well as for educational purposes [5–8]. However, to our knowledge, no studies have been conducted so far for collection of patient data using the iPad prior to radiological examinations. Such an app-based patient briefing could be an alternative to the paper-based version. This could be a feasible way of electronic data capture which might allow instant analysis and postprocessing. This seems highly demanded in an increasingly digitalized world of PACS, RIS, and HIS. Therefore we used the example of patient briefing prior to Magnetic Resonance Imaging (MRI) to demonstrate that a customized iPad app can serve as a digital, user-friendly alternative to conventional paper documentations.
Materials and Methods
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the ethics committee of the University of Erlangen-Nuremberg.
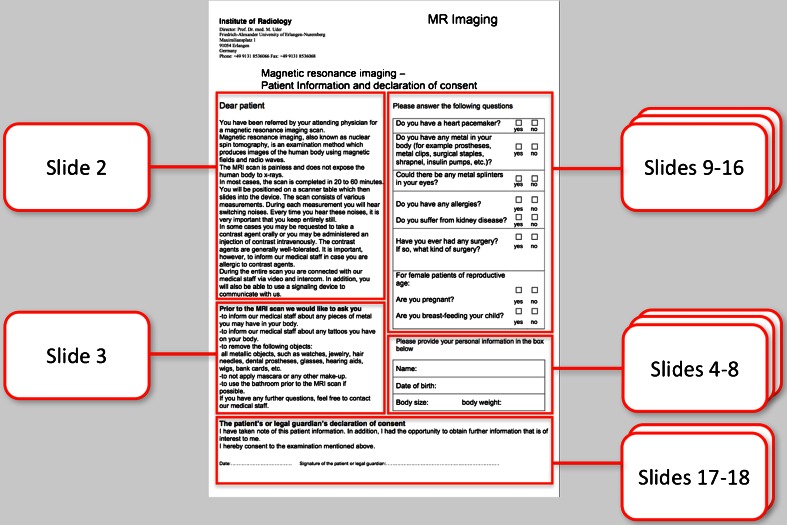
The source document for this study is our standard MRI briefing sheet, which was designed up by our institute and is employed as a paper printout (Fig. 1). The content of the paper briefing was transferred one-to-one to our digital briefing version and converted into an application for the iPad. The development environment, the software developer kit (SDK) for the iPad (Apple Inc., Cupertino, CA, USA), was installed on an iMac® for this purpose and the computer program was created by an expert software programmer within some 70 h using programming language Objective C. A trial version, which is not available on Apple’s AppStore, was subsequently installed on an iPad 2 and used for this study.
Fig. 1.
Conventional MRI briefing in the form of a single A4 sheet of paper
Twenty patients (11 males, 9 females, average age 46.9 years, range 19 to 78 years, standard deviation ± 14.4 years) who visited our institute for MRI, registered at our reception desk, and were asked whether they had already been briefed on the examination and if they were interested to participate in the study. Those patients who answered in the negative and who were willing to participate were included in the study. At first, relevant data on the participants were gathered and noted down separately on a participant list. All participants were then asked to fill in the conventional printout of the briefing document. After a short introduction to the iPad (roughly 5 min), they were then asked to fill in the second, this time electronic, form by themselves. The performers of the study remained in the background and only answered questions if they were asked anything specific. A permanent, active assistance to the patients was not provided. The time the participants needed for each questionnaire was measured and documented by a research assistant. After each participant, the iPad’s surface was cleaned with a surface disinfection agent (Incidin plus®, Ecolab, Düsseldorf, Germany).
The design of the conventional paper questionnaire (Fig. 1), which has been in use for many years, is such that the information text, the questions for risk evaluation as well as the questions on personal data are all on one A4 sheet of paper (210 × 297 mm). The patient reads the text and is asked to answer questions such as any pre-existing illnesses or metal implants by ticking “yes” or “no.” After answering the questions, the patient needs to sign the document in order to give his or her consent to the examination.
As the iPad screen is about 9.7 in. in size (about 24.6 cm) and the patient interacts with the device on a touch screen display using a dedicated pen or his/her finger, the one screen view was—as in the previous publication on the KM Helper—discarded and the content of the classic questionnaire was converted into a sequence model (18 slides; compare to Table 1) with large fonts and symbols (Fig. 2). In order to ensure that both briefing versions can be compared, all texts for the electronic version were adopted one-to-one from the paper-based version; no information was added or removed. The patients were free to decide whether they wanted to operate the iPad with their fingers or with a dedicated input pen (Kensington, Redwood Shores, California, USA).
Table 1.
The briefing app comprises several slides which are retrieved and shown one after another in a path model
| Slide | Fig. | Information | Slide | Fig. | Information |
|---|---|---|---|---|---|
| 1 | 3 | Patient admission and patient list | 10 | Not shown | Metal in body |
| 2 | 4 | Patient Information Part I | 11 | Not shown | Metal splinters in eyes |
| 3 | 5 | Patient Information Part II | 12 | Not shown | Allergies |
| 4 | Not shown | First name | 13 | Not shown | History of kidney disease |
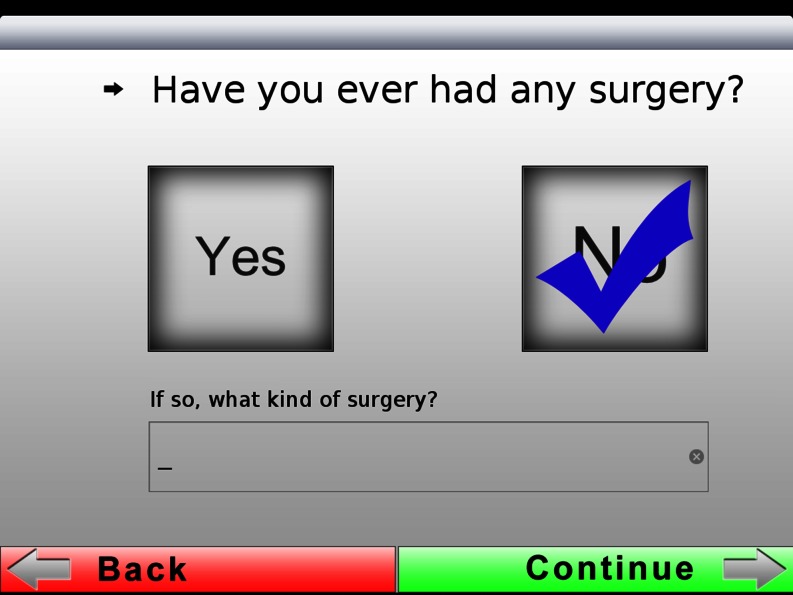
| 5 | 6 | Surname | 14 | Not shown | Previous surgery |
| 6 | 7 | Date of birth | 15 | 9 | Pregnancy |
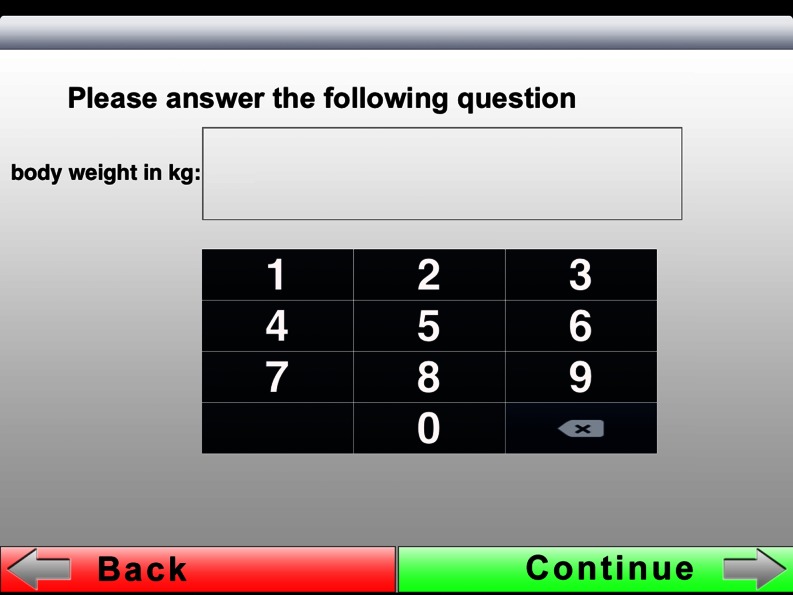
| 7 | 8 | Body weight | 16 | Not shown | Breast feeding |
| 8 | Not shown | Body size | 17 | 10 | Consent |
| 9 | Not shown | Cardiac pacemaker | 18 | 11 | Signature |
For data protection reasons only half the slides were added to this publication
Fig. 2.
The components of the MRI briefing in Fig. 1 are outlined in red and correspond to the content of the 18 slides of the app. Table 1 provides an overview as to which slides of the app are included in the publication
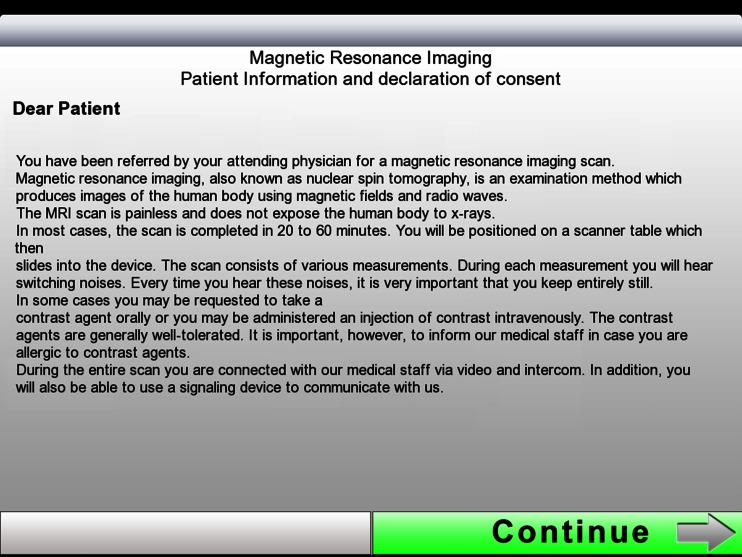
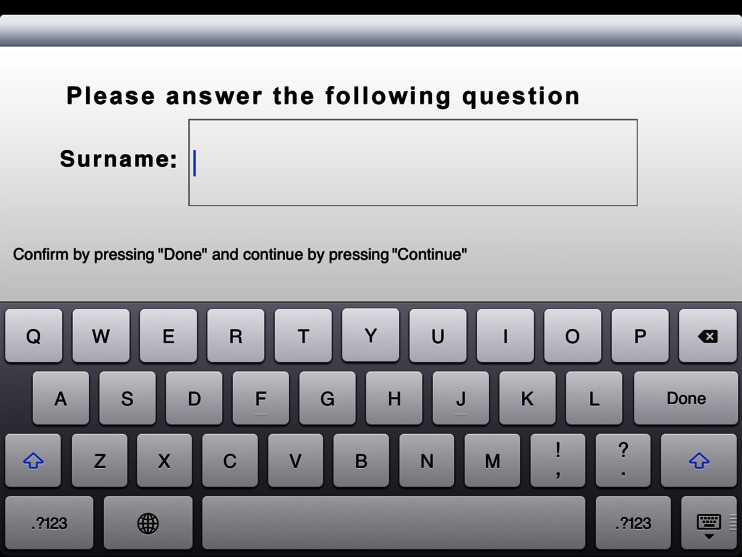
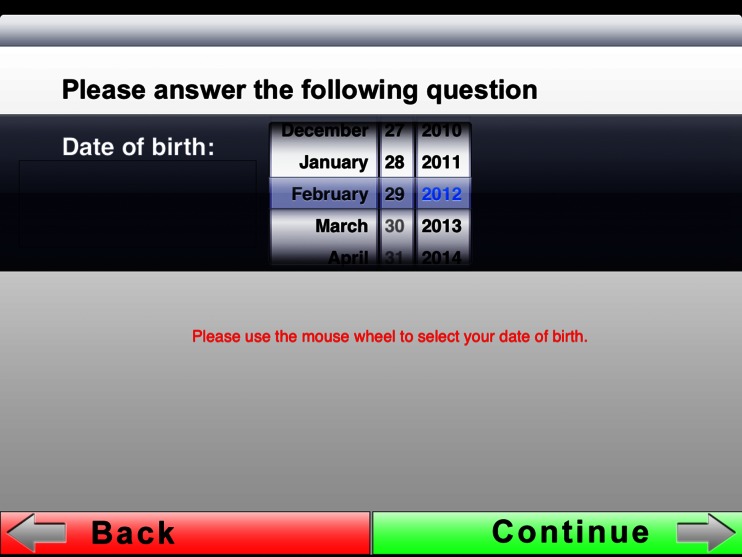
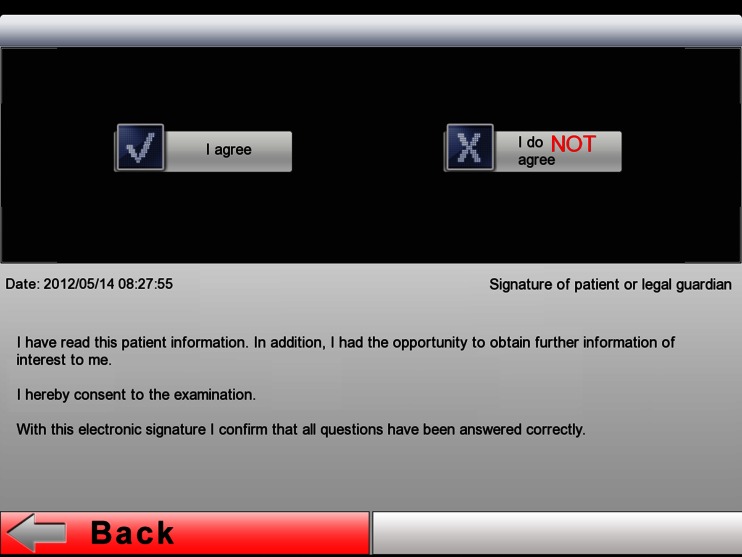
The first slide of the electronic briefing (Fig. 3) displays the options “Patient Admission” and “Patient List.” To register new patients one needs to press “Patient Admission.” “Patient List” shows a list of those patients who have already been briefed, which is why this area is password-protected for data protection reasons. After selecting “Patient Admission,” the first part of the information text appears on the second slide, which provides the user with general information on MRI scanning (Fig. 4). Pressing the “Continue” button shaded in green will take the user to the third slide with the second information text where the patient is asked to prepare for the examination (Fig. 5). After pressing the “Continue” button in the bottom right corner, the input fields on slides 4 and 5 come up where patients are requested to enter their first names and surnames (Fig. 6). These fields are mandatory. After filling in the blanks, the data has to be confirmed by pressing “Done” and the next input fields can be accessed by pressing “Continue.” Pressing “Continue” or “Done” without having entered any text is not possible here. The text is entered using the virtual on-screen standard keyboard, which also allows for the use of special characters. After providing their names and surnames, patients are asked to enter their dates of birth on slide 6. This is done with the help of a scrollbar, which displays the calendar day in numbers, the months as text, and the calendar years as a four-digit number, so that patients do not inadvertently provide any incorrect data. The current date is displayed by default and needs to be adjusted. Otherwise, a red text appears asking the patient to use the scrollbar to choose his or her date of birth (Fig. 7). Afterwards, slides 7 and 8 ask patients about their body weights and body sizes. This data is entered via a numeric keyboard, which is analogous to a cell phone keyboard. Entering weight and size is also mandatory here before patients are allowed to press “Continue” (Fig. 8). On slide 9 to 16 one multiple-choice question per slide has to be answered (compare with Fig. 2). Patients need to choose the correct answer by ticking the respective box (Fig. 9). If the answer concerning previous surgeries is answered with a “yes,” then the patient is required to enter text in the field below. In the cases where the answer is “no” but text has been entered in the field below, a notification appears reminding the patient that the question concerning previous surgeries needs to be answered with a “yes.” He or she will not be able to press “continue” if no information is provided. After answering all questions, the patient will reach the declaration of consent form on slide 17. Here the patient has the option to choose “I agree” or “I do not agree” (Fig. 10). There is no “continue” button on this page because patients are asked to confirm whether they reject or consent to the examination. In addition, the date and time of the briefing are automatically saved and displayed in the screen center on the left-hand side. If the patient does not consent to the examination by pressing “I do not agree,” the briefing process is aborted. If the patient consents and chooses “I agree,” he or she is forwarded to slide 18 and the signature field. In this signature field (Fig. 11), the patient is asked to sign the declaration of consent. A big field was chosen deliberately for this slide in order to facilitate the input of data. Patients can enter data by using either their fingers or the input pen. Large, green arrows make it easier for patients with little iPad experience to sign the declaration by pointing to the touch-sensitive area. After the signing, the briefing is completed and the data is automatically transferred to the archive list. The briefing list can be accessed via the welcome page (Fig. 3) and can only be opened by entering a password. After selecting a patient, the entire briefing is generated as a single page using the conventional design (Fig. 1) and it can be printed via Airprint or cable connection. Alternatively, the briefing data can also be entered into the Radiological Information System (RIS). For data protection reasons, patient data was deleted from the iPad after the data had been gathered, and during the study, the iPad was not connected to a wireless network at any time.
Fig. 3.
The first slide of the briefing. New patient profiles can be created by pressing the button “Patient Admission.” The button “Patient List” leads to the password-protected database in which patient data from prior briefings can be stored. For data protection reasons, this data was deleted after evaluation
Fig. 4.
The second slide of the briefing is used as a patient information leaflet which provides a concise and clear explanation of the examination. The content is identical to the paper briefing
Fig. 5.
The third slide is also used as a patient information leaflet and asks the patient to prepare for the examination. The content is identical to the paper briefing. As with the paper briefing, patients are asked to direct their questions to the medical staff
Fig. 6.
The fifth slide asks patients for their surnames. Patients can enter their names using the virtual standard keyboard in the bottom half of the screen
Fig. 7.
Patients can enter their dates of birth by using scroll wheels, which due to their design, users of smart phones and tablets will be familiar with. To avoid formal misunderstandings concerning days and/or months, the latter were provided in words
Fig. 8.
A numeric keyboard was chosen for providing one’s body weight. The keys were deliberately programmed big so that data can be entered more easily
Fig. 9.
Example of one of the questions: “Have you ever had any surgery?” The answer box is clearly ticked and can be corrected if the wrong box was selected. Specific information can be added in the free text box below
Fig. 10.
For graphical reasons, the declaration of consent field was changed in comparison with the paper briefing, so that patients first need to select the input field by pressing the “I agree“ button in order to get to the signature field
Fig. 11.
The signature field is the 18th and last slide of the briefing. The green arrows point towards the touch-sensitive input field
After signing the document, the patients were asked to return the iPad to the performer of the study who cleaned the surface of the iPad after each turn with a disinfecting agent. Afterwards, participants were asked about their opinion on the electronic briefing. Firstly, the participants were asked how they wish the briefing to be conducted. Possible answers were:
Paper printout.
Electronic version (the way it had just been done).
Electronic version (with additional video and audio material, short films, etc.).
I don’t mind, the important thing is to get it over with quickly.
I have no preference whatsoever.
Patients were given the following options to answer the question as to whether they found the visual version of the electronic briefing appealing:
I strongly agree (1).
I agree (2).
I somewhat agree (3).
I somewhat disagree (4).
I disagree (5).
I strongly disagree (6).
Results
In accordance with the procedure mentioned above, 20 patients were briefed using the paper-based version first and then the electronic version on the iPad.There was no software crash during the briefing. The average processing time for the paper briefing was 2.36 min (range 0.58 to 09.35 min, SD ± 2.05 min); for the iPad version, it was 4.15 min (range 1.56 to 13.48 min, SD ± 2.36 min). Regarding the questionnaire’s content, two questions concerning technical aspects arose during the app-based briefing whereas no question during the paper-based briefing came up (Table 2).
Table 2.
Average time participants needed to process the documents of the patient briefing
| Printed briefing | Electronic briefing | |
|---|---|---|
| Processing time (min) | 2:36 | 4:15 |
| Questions | 0 | 2 |
General questions were asked after the briefing with regard to the documented patient briefing. Patients answered the question as to what kind of briefing they preferred for the future six times with two (electronic version, the way it had just been done), whereas this was the case in only four patients concerning the paper printout (answer 1). None of our patients wished additional multimedia information (answer 3). Eight patients did not have any general preference in regard to the paper-based or electronic version but five out of these eight preferred the quicker version; no matter what medium was used, three patients had no preference whatsoever. Two participants did not answer the question.
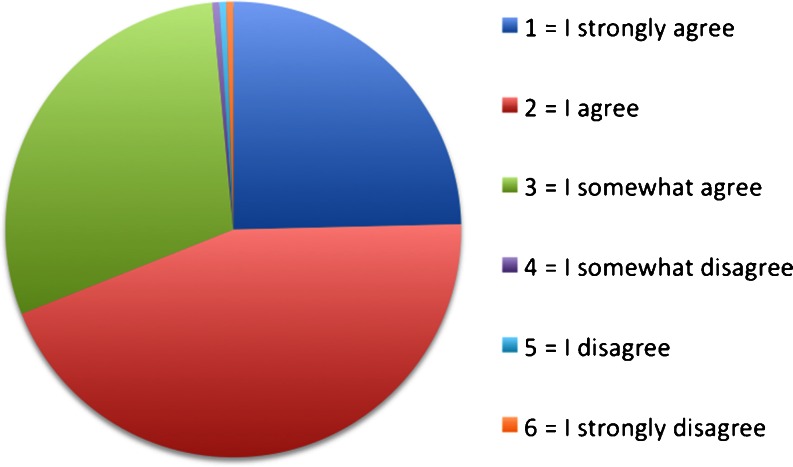
Patients answered the question as to whether they found the electronic briefing visually appealing 5 times with 1 (I strongly agree), 9 times with 2 (I agree), 6 times with 3 (I somewhat agree), 0 times with 4, 5 or 6 (I somewhat disagree, I disagree, I strongly disagree; Fig. 12).
Fig. 12.
Evaluation of the visual version (“I find the app visually appealing”)
Discussion
The purpose of this study was to evaluate the feasibility and user friendliness of an iPad application-based patient briefing for MRI. Prior to many radiological examinations, a documented patient briefing has to be conducted in order to determine whether any contraindications for the examination or a heightened risk for undesired reactions to drugs exist. Several authors have dealt with the issue of documented patient briefings in recent years. Their focus of analysis revolved around the use of media as a supplement to the conventional paper briefing in order to fully inform patients about all planned medical procedures and to document their consent to the treatment. This way it was possible to lower the anxiety levels of patients before they had heart surgery and also to increase their tolerability and satisfaction scales with the help of video-assisted patient briefings [9]. In a similar study, it was found that, prior to arthrodesis surgery, it was possible to increase the patients’ understanding of the risks, the advantages and the importance of postoperative treatment with the help of a multimedia education tool [10]. In general, however, there still seems to be a great deal of demand for goal-oriented information on the part of patients [11]. This could be satisfied in the form of digital briefings via modern tablets. In a previous article on the “KM Helper,” a training tool for smart phones which helps to evaluate the risk of unwanted reactions to drugs prior to intravenous or intraarterial injections of iodine-containing contrast agents, the authors had already shown that smart phone apps can have a virtually universal employability in a hospital’s everyday work life [12]. With respect to the patient briefing, however, the small-sized screens of smart phones lead to the fact that their employability is rather limited, which will certainly not be tolerated by a majority of patients, especially those with impaired sight. This limitation can be overcome by using modern tablets such as Apple’s iPad due to its much larger screen.
Our preliminary results show that there is no difference regarding the completeness of data acquisition and that the majority of patients seem to prefer the app-based approach for future briefings. The electronic version of the patient briefing, which has the same information content and the same number of questions, takes on an average of 1.39 min longer to complete than the paper-based version. A discussion of this point appears difficult, as no studies dealing with the difference in average processing time of paper and electronic briefings could be found in PubMed (status as of July 01, 2012). A reason for this longer duration of the electronic briefing may be found in the composition of the 18 slides in this sequence model. As patients had to answer one question before the next one was displayed and could be answered, this may be the reason for the fact that more time was invested in this briefing version. Another influencing factor may be that not all study participants were familiar with the use of electronic devices such as the iPad. One participant took 13.48 min to complete the electronic briefing, which on average required a processing time of 4.15 min. A follow-up study should analyze to what extent individual factors such as patient age, education, profession, or IT knowledge have an influence on handling the electronic briefing.
Regarding all theses possible explanations for the longer duration of the electronic briefing (4.14 versus 2.36 min), we strongly believe that patients might read digital text more thoroughly. On printed sheets, information might be skipped more easily and the patient may continue with the questions directly. As in the electronic version, the patient can only proceed with the briefing by active confirming the understanding of the information of each slide (“Continue” or “Done” buttons) he/she might be “forced” to read the text (Figs. 4 and 5). We deduce from this that the electronic version may take more time, but is just as suitable or an even better approach for the documented patient briefing as the paper-based type.
This theoretical benefit of iPad-based briefing has to be confirmed in greater detail on a larger patient collective. In follow-up studies, it should be analyzed too whether this model is universally deployable or if the electronic version is suitable for certain patient groups only.
If the electronic version becomes employable on a large scale, it would be desirable to send patients the electronic patient briefing early on, facilitate the recognition of problematic constellations as, e.g., history of renal disease, metal implants, and allergies, early in advance. However, this must not be a substitute for personal one-on-one consultations. Moreover, it is important to note that country-specific legal frameworks, such as for example, data protection guidelines, need to be taken into consideration when the electronic briefing is used.
An advantage of the iPad-based briefing could be found in the fact that the information from completed patient forms could be added more easily and quickly to a digital patient portfolio in the RIS or other databases. This for example would allow the automatic generation of warnings such as “caution, patient has a cardiac pacemaker.”
Moreover the sometimes hard to read handwriting of patients on conventional forms can be avoided using electronic devices.
Furthermore, due to the digital form of the electronic briefing, there would also be the option to make it more attractive for patients by adding multimedia content, such as informative films, as well as to increase the understanding among patients of the disease and the examination [10, 11, 13]. Interestingly in our study collective no participant has expressed interest in a briefing with more interactive content, such as pictures, audio material or informative films. It seems that the majority of patients wish compact information which is supported by the fact that 25 % of our patients preferred the quickest approach (Table 3).
Table 3.
Number of wishes expressed regarding preference for a certain type of patient briefing
| Briefing version | Number of wishes |
|---|---|
| Paper printout | 4 |
| Electronic form | 6 |
| Electronic form (including video and audio material, short film etc.) | 0 |
| I don’t mind, the important thing is to get it over with quickly | 5 |
| No preference whatsoever | 3 |
| No answer | 2 |
Some limitation needs to be addressed. All participants first performed the paper-based patient briefing followed by the iPad-based version. This possibly influences the results; however, in this study, we focused on the feasibility of the app-based method during the clinical routine and on the acceptance by the patients. The number of study participants needs to be increased for further investigations.
In our opinion, modern tablets such as the iPad are a promising technology for medical purposes due to their simple user interface and broad employability. With the right design (e.g., large fonts and easy to understand symbols), they seem suitable for a wide patient spectrum and can serve as a versatile alternative to paper-based patient briefing before radiological examinations.
Footnotes
Philipp Martin Schlechtweg and Matthias Hammon contributed equally to this work.
References
- 1.Tsukayama H: Apple’s record iPad sales, in context. The Washington Post. 2012. Available at: http://www.washingtonpost.com/business/technology/apples-record-ipad-sales-in-context/2012/03/20/gIQAaxDYPS_story.html. Accessed June 17, 2012.
- 2.Dolan B: Types of medical apps the FDA will regulate | mobihealthnews. Mobihealthnews. 2011. Available at: http://mobihealthnews.com/11980/types-of-medical-apps-the-fda-will-regulate/. Accessed August 4, 2012.
- 3.Dolan B: How FDA and FTC co-regulate health apps | mobihealthnews. Mobihealthnews. 2012. Available at: http://mobihealthnews.com/16729/how-fda-and-ftc-co-regulate-health-apps/. Accessed August 4, 2012.
- 4.Health C for D and R. Products and Medical Procedures - Mobile Medical Applications. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ucm255978.htm. Accessed August 4, 2012.
- 5.Mc Laughlin P, Neill SO, Fanning N, et al. Emergency CT brain: preliminary interpretation with a tablet device: image quality and diagnostic performance of the Apple iPad. Emerg Radiol. 2012;19(2):127–133. doi: 10.1007/s10140-011-1011-2. [DOI] [PubMed] [Google Scholar]
- 6.John S, Poh ACC, Lim TCC, Chan EHY, Chong LR: The iPad Tablet Computer for Mobile On-Call Radiology Diagnosis? Auditing Discrepancy in CT and MRI Reporting. Journal of digital imaging: the official journal of the Society for Computer Applications in Radiology. 2012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22562174. Accessed May 30, 2012. [DOI] [PMC free article] [PubMed]
- 7.Sadri A, Murphy AD, Odili J: iPad local flap pre-operative planning: A good training tool. Journal of plastic, reconstructive & aesthetic surgery: JPRAS. 2012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22578789. Accessed May 30, 2012. [DOI] [PubMed]
- 8.Richardson ML, Petscavage JM, Hunter JC, Roberts CC, Martin TP. Running an Online Radiology Teaching Conference: Why It’s a Great Idea and How to Do It Successfully. Acad Radiol. 2012;19(6):746–751. doi: 10.1016/j.acra.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Philippe F, Meney M, Larrazet F, et al. Effects of video information in patients undergoing coronary angiography. Arch Mal Coeur Vaiss. 2006;99(2):95–101. [PubMed] [Google Scholar]
- 10.Beamond BM, Beischer AD, Brodsky JW, Leslie H. Improvement in surgical consent with a preoperative multimedia patient education tool: a pilot study. Foot Ankle Int. 2009;30(7):619–626. doi: 10.3113/FAI.2009.0619. [DOI] [PubMed] [Google Scholar]
- 11.Mulsow JJW, Feeley TM, Tierney S. Beyond consent–improving understanding in surgical patients. Am. J. Surg. 2012;203(1):112–120. doi: 10.1016/j.amjsurg.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Schlechtweg PM, Kuefner MA, Heberlein C, et al. A useful tool for routine radiological examinations: the iPhone application “KM Helper”. Radiologe. 2011;51(5):392–396. doi: 10.1007/s00117-011-2169-z. [DOI] [PubMed] [Google Scholar]
- 13.Eggers C, Obliers R, Koerfer A, et al. A multimedia tool for the informed consent of patients prior to gastric banding. Obesity (Silver Spring) 2007;15(11):2866–2873. doi: 10.1038/oby.2007.340. [DOI] [PubMed] [Google Scholar]