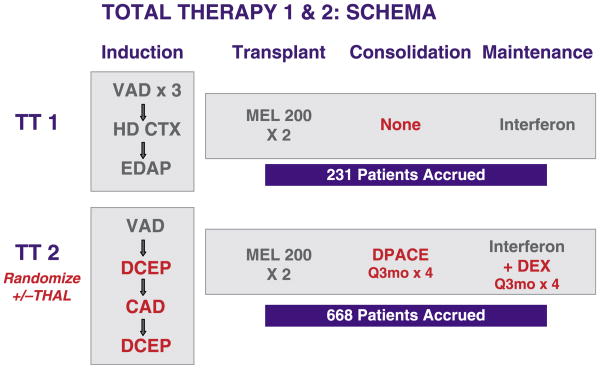
Fig 1.
Treatment schemata for total therapy 1 (TT1) and total therapy (TT2). TT1 was a phase II trial whereas TT2 was a phase III trial with a control arm and an experimental arm that added thalidomide (THAL) from the initiation of therapy. In comparison to TT1, TT2 employed more intensive haematopoietic growth factor-requiring induction chemotherapy (DCEP, CAD) and introduced post-transplant consolidation chemotherapy with DPACE, administered every 3 months for four cycles. Maintenance therapy in TT2 added high-dose dexamethasone pulsing every 3 months for four cycles to interferon maintenance, which was then continued as single agent until relapse or undue toxicity. For details of regimens and abbreviations, refer to text.