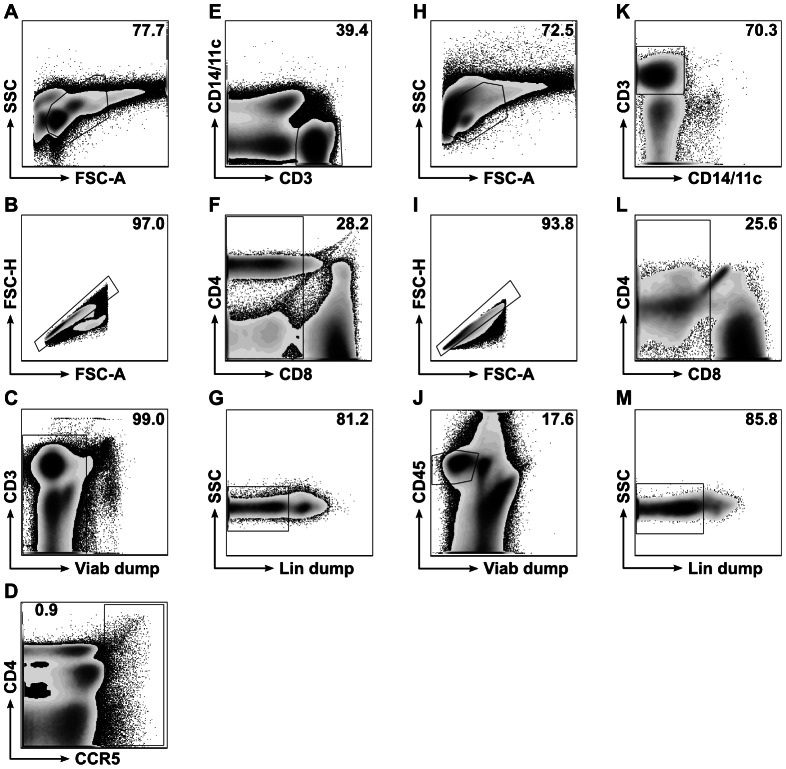
Figure 2. Fluorescence-activated cell sorting (FACS) strategies for PBMC samples (A-G) and GI tract samples (H-M; ileum shown as example).
For PBMC, all cells were included (panel A), doublets excluded (panel B), and residual non-viable cells excluded by LIVE/DEAD violet cell staining ("Viab dump," panel C). Low-frequency CCR5+ events were then collected in one sorting tube (inside box gate, panel D). Of the remaining, CCR5- events (outside box gate, panel D), CD3+ events negative for CD14 and CD11c were included for further gating (inside polygon gate, panel E), with remaining events collected in a second sorting tube (outside polygon gate, panel E). CCR5-CD3+ events negative for CD14 and CD11c that were also CD8- (panel F) and T cell receptor-γδ-, CD20-, and CD56- ("Lin dump," panel G) were collected in a third sorting tube as presumptive CD4+ T cells. Remaining CD3+ events that were either CD8+ or Lin dump+ were combined in a fourth sorting tube. For ileum and rectum, all cells were included (panel H) and then doublets excluded (panel I). Viable CD45+ events were included for further gating (inside polygon gate, panel J), with all events outside this gate collected in one sorting tube as non-hematopoietic cells. CD3+ events negative for CD14 and CD11c were included for further gating (inside polygon gate, panel K), with remaining events collected in a second sorting tube (outside polygon gate, panel K). CD3+ events negative for CD14 and CD11c that were also CD8- (panel L) and T cell receptor-γδ-, CD20-, and CD56- ("Lin dump," panel M) were collected in a third sorting tube as presumptive CD4+ T cells. Remaining CD3+ events that were either CD8+ or Lin dump+ were combined in a fourth sorting tube. Numbers in upper-right corners of flow plots indicate the percentages of events on plots falling inside gates shown.