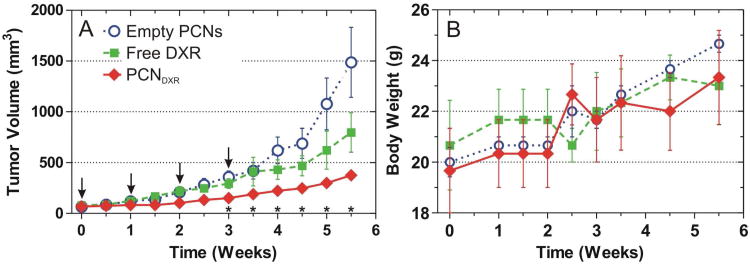
Figure 5.
In vivo antitumor effects of PCNDXR, empty PCNs, or free DXR as administered by intraperitoneal injection to female nude mice bearing MDA-MB-231 human triple negative mammary tumors (n = 3 mice with bilateral tumors, 6 tumors per group). (A) Mean tumor volume. Pairwise tests were performed to assess statistical significance and were Bonferroni-corrected; * indicates p < 0.05 for PCNDXR compared with empty PCNs. PCNDXR was also significantly different from free DXR on weeks 1.5 and 2.5 (not shown in plot). Times of treatments are indicated by arrows (points, raw mean; error bars, standard error). (B) Body weights of each treatment group (points, mean; error bars, standard error).