Abstract
Objectives
Measuring urinary cotinine is a popular and established method of biologically monitoring exposure to tobacco smoke. However, the lower detection limit of cotinine often impedes the evaluation of passive (second-hand) smoking and this, together with unconverted nicotine, does not reflect actual levels of exposure. Furthermore, a portion of the Japanese population might have decreased ability to metabolize nicotine. The present study was therefore carried out to validate the simultaneous analysis of total concentrations of free nicotine and cotinine and their glucuronides to determine actual levels of voluntary and involuntary exposure to cigarette smoke.
Methods
Urine samples from 118 Japanese smokers and 117 non-smokers were analyzed using gas chromatography–mass spectrometry. Voluntary and involuntary smoking status was self-reported and workplace smoking restrictions were objectively evaluated.
Results
The integrated sum of all concentrations showed 2.2- and 2.4-fold higher total levels (free and glucuronide) of nicotine and cotinine relative to the free levels. Median (quartiles) of total nicotine and cotinine were 1635 (2222) and 3948 (3512) ng/mL in smokers, and 3.5 (5.3) and 2.8 (4.2) ng/mL in non-smokers. Concentrations of urinary nicotine were higher than those of cotinine in 21 % of smokers and in 54 % of non-smokers. Nicotine and cotinine levels were significantly associated with a smoking habit, as well as being significantly associated with the workplace and home environments of non-smokers.
Conclusions
The present method can monitor voluntary and involuntary exposure to tobacco smoke. Measuring total urinary nicotine levels might be useful for analyzing exposure to cigarette smoke among non-smokers.
Keywords: Tobacco smoke, Nicotine, Cotinine, GC–MS, Japanese
Introduction
Cotinine can be detected in various biological samples including urine, blood, hair, and saliva. Measuring cotinine concentrations in such samples is the most popular methodology for evaluating exposure to tobacco smoke [1]. Various chromatographic techniques (e.g., ultraperformance liquid chromatography [UPLC], liquid chromatography [LC]/mass spectrometry [MS]/MS, gas chromatography–mass spectrometry [GCMS]) and immunoassays [2–6] have been applied to determine exposure to tobacco smoke, and some of these methods have also been applied practically in epidemiological studies [7–10]. For example, Yuan and colleagues found that urinary cotinine levels were associated with lung cancer development in smokers [8]. In addition, low levels of urinary cotinine resulting from exposure to second-hand smoke are inversely correlated with respiratory function [10].
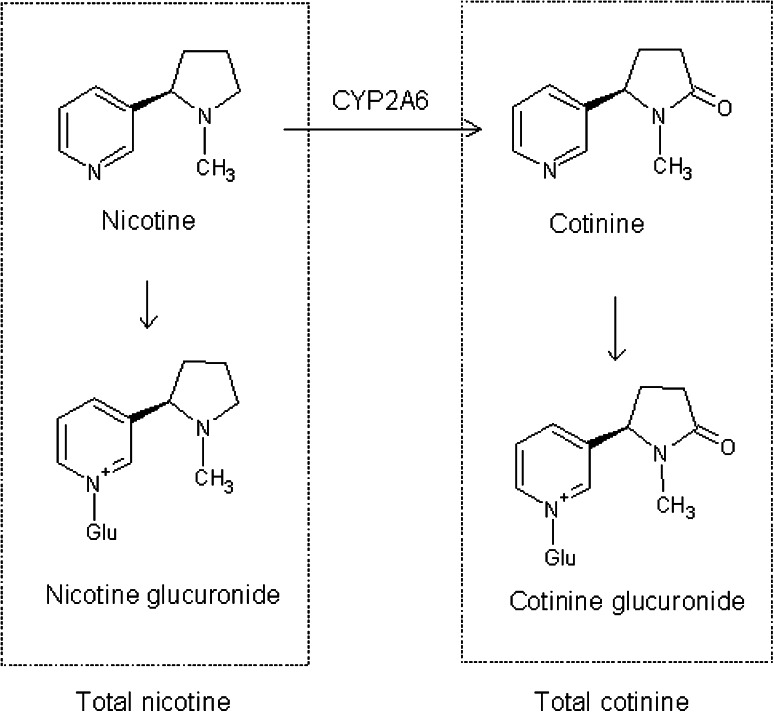
Nicotine, cotinine, and their glucuronides are the major chemical species that are detectable after exposure to cigarette smoke (Fig. 1). In the present study we analyzed urine samples from 235 Japanese workers including 117 non-smokers to validate our analytical procedure and to examine correlations between urinary nicotine and cotinine values and smoking (active and passive).
Fig. 1.
Nicotine metabolites. Around 80 % of nicotine is oxidized to cotinine, mainly by cytochrome P450 2A6 (CYP 286) [19, 20]. Nicotine and cotinine glucuronides were hydrolyzed to nicotine and cotinine and measured as total nicotine and cotinine (sum of free compound and glucuronides)
A relatively short half-life (a few hours) has rendered measuring nicotine levels of little value in terms of assessing exposure to cigarette smoke. In contrast, the half-life of cotinine is approximately 20 h [11]. Here, we simultaneously measured nicotine and cotinine because the procedure is straightforward [12, 13] and half of all Japanese individuals have low activity of cytochrome P450 2A6 (CYP2A6), which is needed to metabolize nicotine to cotinine [14]. Nicotine detection might be useful when individuals have less active CYP2A6 phenotypes.
Although the measurement of total cotinine concentrations is not typical, a few research groups have applied the technique [5, 8, 15]. Total measurements confer an obvious advantage in that the signal is greater relative to that of free concentrations. The method has been applied with the expectation of a closer correlation with actual exposure rather than that gained by simply measuring levels of free cotinine, as we have previously shown [5].
Subjects, materials, and methods
Study population
Urine samples were collected at a facility that routinely performs medical examinations at workplaces in Saga, Japan. All donors provided written informed consent to participate in the study, the design of which was approved by the Medical Ethics Committee of Saga Medical School.
Thirty-five spot urine samples, collected from 16 employees aged 21–59 years between 2001 and 2003, were stored at −20 °C. All of the participants were actively participating in a smoking cessation program.
Another group of spot urine samples taken from 219 individuals (age range, 18–60 years) in 2008 and stored at −20 °C were analyzed in 2008. Information regarding the smoking habits of the participants and their family members was obtained from questionnaires administered at the time of sample collection. The study participants were employed by 11 subcontractors of a manufacturing company. The subcontractors were located at adjacent sites but each had different policies regarding smoking in the workplace and in the immediate environment surrounding the workplace. Smoking restriction status was verified by interviews and inspections by an occupational physician. Among the 219 individuals in this group, 117 were non-smokers (female, n = 59) and 102 were smokers (female, n = 16). The subcontractors were engaged in various industries including ironworks and transport.
Sample analysis
Sample preparation and GC–MS analysis proceeded as described [5]. Diphenylamine (internal standard), nicotine, and cotinine were quantified by monitoring peaks at 51.05/169.1, 162.15/161.5, and 176.10/98.1, respectively. This method demonstrated good linearity (r = 0.99 for both nicotine and cotinine) with the following lower limits of detection and of quantification (LLOQ) (3 and 10 standard deviations each) of 0.4 and 1.3 ng/mL for nicotine, and 0.2 and 0.7 ng/mL for cotinine, respectively.
Creatinine levels were measured using a Creatinine Assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) to normalize urine concentrations.
Results
Comparison of free and total values
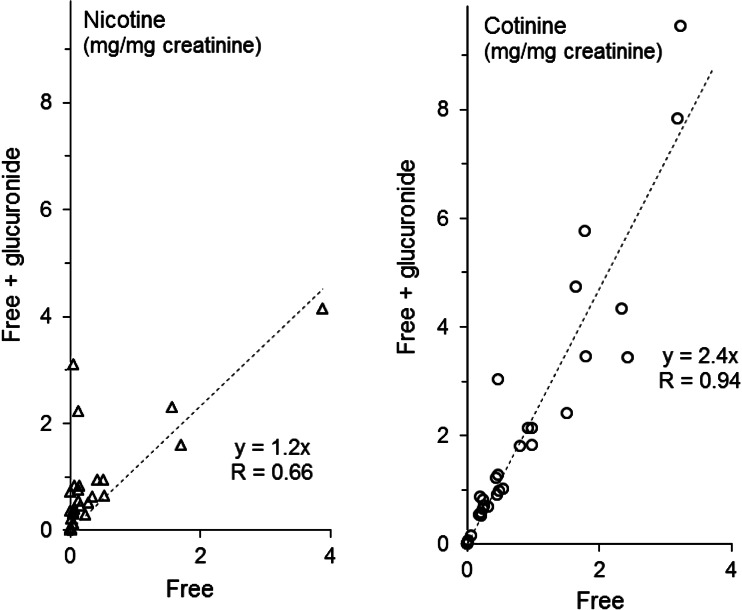
Regression coefficients indicated that total nicotine and cotinine concentrations were 1.2- and 2.4-fold higher relative to the free values (Fig. 2). The integrated sum of all concentrations revealed 2.2- and 2.4-fold higher total levels of nicotine and cotinine relative to the free levels. When free nicotine was measured, eight samples were below the LLOQ, while one sample was below the LLOQ for cotinine measurement. In comparison, two and zero samples were below the LLOQ when total nicotine and total cotinine, respectively, were evaluated.
Fig. 2.
Free and total nicotine and cotinine levels in human urine. Values in 35 spot urine samples from 16 employees analyzed without/with de-glucuronidation (free/total). Median (quartiles) of free and total nicotine were 50 (192) and 359 (768) μg/mg creatinine, and 442 (862) and 901 (1928) μg/mg creatinine for free and total cotinine, respectively
Total nicotine and cotinine levels in 219 employees
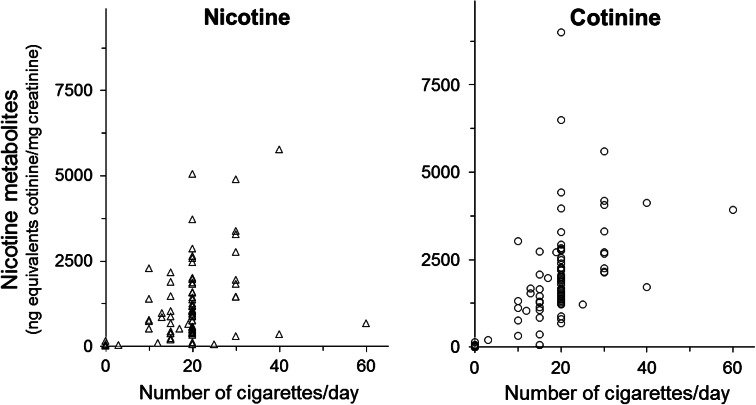
Smokers showed higher nicotine and cotinine levels than non-smokers (Table 1). Among smokers, male subjects showed significantly higher nicotine and cotinine levels than female subjects, whereas there was no clear difference between the sexes among non-smokers (Table 1). The secretion ratios of nicotine and cotinine differed significantly between smokers and non-smokers (Table 1). Scatter plots of individual distributions showed that 21 % of smokers and 54 % of non-smokers had greater urinary nicotine than cotinine levels (nicotine:cotinine >1) (Fig. 3). Moreover, 5 and 37 % of smokers and non-smokers, respectively, had nicotine levels that were two or more times greater than cotinine levels (nicotine:cotinine >2). The accumulated sum of all samples showed 1.8-fold higher cotinine than nicotine levels in smokers, and the same levels of both in non-smokers. Both nicotine and cotinine concentrations were strongly correlated with the numbers of cigarettes smoked per day (Fig. 4).
Table 1.
Total nicotine and cotinine concentrations in urine samples from Japanese employees
| All |
N 219 |
Nicotine | Cotinine | Nicotine/cotinine ratio | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ng/mL) | (ng/mg creatinine) | (ng/mL) | (ng/mg creatinine) | (moles/moles) | |||||||||||||
| Median | Quartile | Median | Quartile | Median | Quartile | Median | Quartile | Median | Quartile | ||||||||
| Smokers | 102 | 1635.2 | 2222.2 | 804.4 | 1170.6 | 3948.1 | 3512.2 | 1984.3 | 1468.8 | 0.42 | 0.68 | ||||||
| Non-smokers | 117 | 3.5 | 5.3 | 3.8 | 5.8 | 2.8 | 4.2 | 2.7 | 5.2 | 1.22 | 1.86 | ||||||
| pa | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
| Male | 146 | pb | pb | pb | pb | pb | |||||||||||
| Smokers | 86 | 1756.7 | 2177.3 | <0.001 | 857.4 | 1156.4 | 0.018 | 4698.4 | 3225.5 | 0.002 | 2029.8 | 1467.9 | 0.076 | 0.47 | 0.68 | 0.178 | |
| Non-smokers | 60 | 2.9 | 6.0 | 0.433 | 3.2 | 5.4 | 0.040 | 3.7 | 6.8 | 0.062 | 3.2 | 7.3 | 0.663 | 0.82 | 1.78 | 0.010 | |
| pa | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
| Female | 63 | ||||||||||||||||
| Smokers | 16 | 766.7 | 1021.3 | 490.2 | 749.6 | 2386.1 | 2431.4 | 1648.8 | 1495.7 | 0.25 | 0.43 | ||||||
| Non-smokers | 57 | 4.2 | 4.8 | 4.9 | 5.8 | 2.4 | 2.9 | 2.6 | 2.6 | 1.48 | 1.97 | ||||||
| pa | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
Differences determined by t-test using logarithms; 0.28 ng/mL, detection limit of nicotine/√2 was applied to data below detection limits (5 male and 3 female non-smokers). All cotinine values were above detection limits
N number of participants
aSmokers versus non-smokers
bMales versus females
Fig. 3.
Differences in nicotine and cotinine concentrations between smokers and non-smokers. Data are shown as total (free + glucuronide) values. N = 102 and 117 for smokers and non-smokers, respectively. More non-smokers showed a high nicotine-to-cotinine ratio than smokers. Dotted line shows y = x (for reference)
Fig. 4.
Correlations between number of cigarettes smoked per day and nicotine metabolite concentrations in 219 urine samples. Data are shown as total (free + glucuronide) values. Spearman’s rank correlation coefficients (p value) for nicotine and cotinine were 0.84 (<0.0001) and 0.85 (<0.0001), respectively
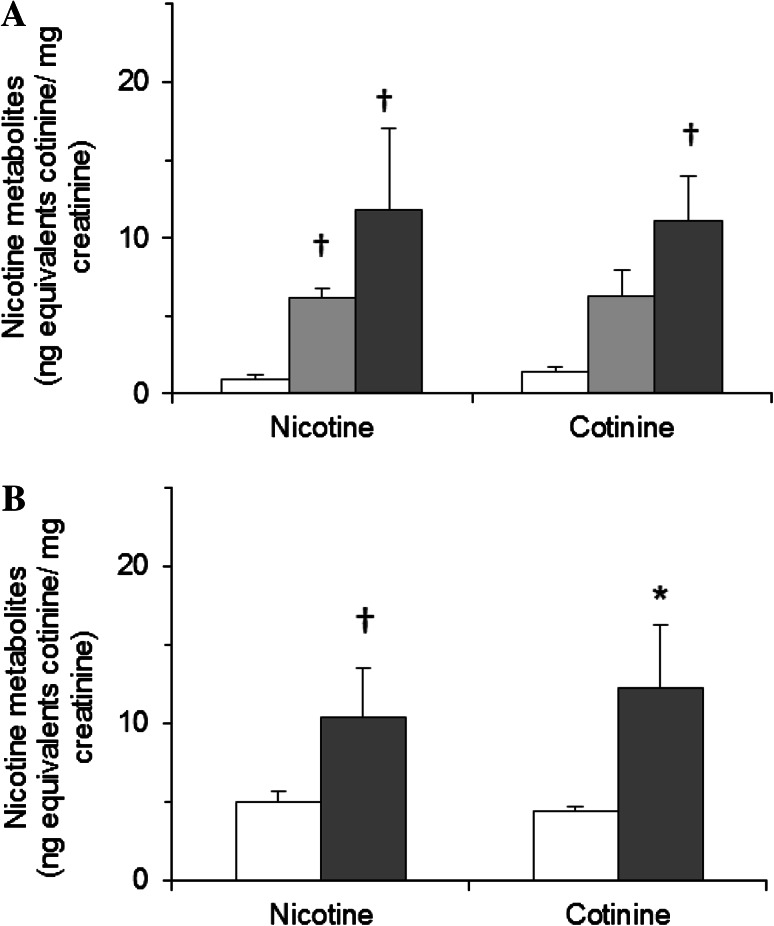
Data from non-smokers were obviously affected by workplace policies prohibiting smoking and whether or not anyone smoked at home. Nicotine values indicated a clearer environmental effect than cotinine alone (Fig. 5).
Fig. 5.
Correlation between passive smoking and urinary nicotine metabolite concentrations in non-smokers. Results show total concentrations (free + glucuronide); *p < 0.01, †p < 0.001, significant difference compared with groups with lowest values by t-test with logarithm. a Smoking at workplace: prohibited (open bars, n = 6), partially prohibited (gray bars, n = 88), permitted (black bars, n = 23). b Smokers at home (black bars, n = 39), no smokers at home (open bars, n = 70). Answers to the questions about smoking conditions at home and in the workplace were not obtained from 8 participants
Discussion
The present study showed that the simultaneous analysis of total nicotine and cotinine was useful for biologically monitoring active and passive smoking. Nicotine and cotinine were simply detected with a single GC–MS run [12]. Although an extra hour was required to deglucuronidate urine samples, total values of urinary nicotine and cotinine were high, which was particularly advantageous for analyzing samples from non-smokers.
Total nicotine and cotinine were closely correlated with active and passive smoking. The present protocol confers less benefit upon analyses of urine from smokers, because levels of metabolites are high enough to detect, and the main metabolite cotinine reflects smoking status more accurately than nicotine. In contrast, average urinary nicotine and cotinine levels were the same in non-smokers, and some samples contained high nicotine and low cotinine levels, indicating that simultaneous measurement generates meaningful data from non-smokers.
Benowitz and colleagues showed that 7 and 6 % of nicotine and its glucuronide, respectively, were excreted and 17 and 15 % of cotinine and its glucuronide, respectively, were excreted within 24 h [19]. Consequently, the theoretical total nicotine/total cotinine ratio is 0.4, which is compatible with the present median finding in smokers of 0.42. However, in the present study, the median nicotine/cotinine ratio of 1.22 was significantly higher in non-smokers than smokers. A study of 959 urine samples from Malaysian university students also found significantly higher nicotine levels in non-smokers than in smokers [13].
The explanation for this might be associated with the half-lives of nicotine and cotinine and the time of urine collection. For example, if the employees in our study had been exposed to second-hand tobacco smoke in the workplace within a few hours before the urine sampling, the nicotine concentration in their urine would have risen because the nicotine would not yet have been metabolized to cotinine. An increased nicotine concentration would have a big impact in urine samples from non-smokers, but not in the urine samples from smokers, because they would already have had high concentrations of nicotine and cotinine in their urine.
Different CYP2A6 polymorphisms among individuals of different ethnicity provide another possible reason for significantly higher nicotine levels in non-smokers than in smokers. According to Nakajima and colleagues, 9, 22, 43, and 51 % of Caucasian, Black, Korean, and Japanese populations have a CYP2A6 polymorphism that results in slow nicotine metabolism [14]. Because CYP2A6 activity correlates with cigarette consumption [16–18], Asian non-smokers might have lower CYP2A6 activity than non-smokers in other ethnic groups.
Thus, the present findings indicate that the simultaneous analysis of total nicotine and cotinine is simple and useful for tobacco smoke monitoring especially for non-smokers with lower CYP2A6 activity. However, a major limitation of this study is the lack of genotype information. The association between the usefulness of nicotine monitoring and CYP2A6 polymorphism needs to be proven by further research involving actual genotyping. Another concern might be the volatility of nicotine. As urine samples were incubated at 75 °C for 1 h for deglucuronidation, artifactual loss could have occurred more in nicotine than in cotinine, based on Henry’s Law constant (3.0 × 10−9 and 3.3 × 10−12 atm- m3/mole, respectively) (http://www.ncbi.nlm.nih.gov/pccompound). We previously reported that adjusting urinary cotinine values according to the creatinine concentration was possibly ineffective with urine samples from children [5]; however, in the present study, the creatinine-adjusted nicotine and cotinine concentrations showed stronger correlation with active/passive smoking status than the unadjusted data, which indicates the adjustment is meaningful with adult urine.
In conclusion, the simultaneous measurement of total urinary nicotine and cotinine is useful particularly among non-smokers with higher urinary nicotine than cotinine, such as individuals with CYP2A6 polymorphisms.
Acknowledgments
This study was funded by a grant from the Japanese Ministry of Health, Labour and Welfare (Clinical Cancer Research Project). The authors are grateful to Ms. Ranko Nishi and Mr. Tatsuya Takahashi for their technical assistance.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. A report of the surgeon general; the health consequences of involuntary exposure to tobacco smoke. Pittsburgh, PA: U.S. Government Printing Office: 2006. [PubMed]
- 2.Sasaki S, Braimoh TS, Yila TA, Yoshioka E, Kishi R. Self-reported tobacco smoke exposure and plasma cotinine levels during pregnancy—a validation study in Northern Japan. Sci Total Environ. 2011;412–413:114–118. doi: 10.1016/j.scitotenv.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Miller EI, Murray GJ, Rollins DE, Tiffany ST, Wilkins DG. Validation of a liquid chromatography-tandem mass spectrometry method for the detection of nicotine biomarkers in hair and an evaluation of wash procedures for removal of environmental nicotine. J Anal Toxicol. 2011;35:321–332. doi: 10.1093/anatox/35.6.321. [DOI] [PubMed] [Google Scholar]
- 4.Shin HS, Kim JG, Shin YJ, Jee SH. Sensitive and simple method for the determination of nicotine and cotinine in human urine, plasma and saliva by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;769:177–183. doi: 10.1016/S1570-0232(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto A, Ino T, Ohta M, Otani T, Hanada S, Sakuraoka A, et al. Enzyme-linked immunosorbent assay of nicotine metabolites. Environ Health Prev Med. 2010;15:211–216. doi: 10.1007/s12199-009-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CF, Uang SN, Chiang SY, Shih WC, Huang YF, Wu KY. Simultaneous quantitation of urinary cotinine and acrylonitrile-derived mercapturic acids with ultraperformance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2012;402:2113–2120. doi: 10.1007/s00216-011-5661-4. [DOI] [PubMed] [Google Scholar]
- 7.Leenders M, Chuang SC, Dahm CC, Overvad K, Ueland PM, Midttun O, et al. Plasma cotinine levels and pancreatic cancer in the EPIC cohort study. Int J Cancer 2011;131:997–1002. [DOI] [PubMed]
- 8.Yuan JM, Gao YT, Murphy SE, Carmella SG, Wang R, Zhong Y, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71:6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. 2011;165:741–748. doi: 10.1001/archpediatrics.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai HK, Hedley AJ, Repace J, So C, Lu QY, McGhee SM, et al. Lung function and exposure to workplace second-hand smoke during exemptions from smoking ban legislation: an exposure-response relationship based on indoor PM2.5 and urinary cotinine levels. Thorax. 2011;66:615–623. doi: 10.1136/thx.2011.160291. [DOI] [PubMed] [Google Scholar]
- 11.Ahijevych KL, Tyndale RF, Dhatt RK, Weed HG, Browning KK. Factors influencing cotinine half-life during smoking abstinence in African American and Caucasian women. Nicotine Tob Res. 2002;4:423–431. doi: 10.1080/1462220021000018452. [DOI] [PubMed] [Google Scholar]
- 12.Man CN, Gam LH, Ismail S, Lajis R, Awang R. Simple, rapid and sensitive assay method for simultaneous quantification of urinary nicotine and cotinine using gas chromatography–mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:322–327. doi: 10.1016/j.jchromb.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Man CN, Fathelrahman AI, Harn GL, Lajis R, Samin AS, Omar M, et al. Correlation between urinary nicotine, cotinine and self-reported smoking status among educated young adults. Environ Toxicol Pharmacol. 2009;28:92–96. doi: 10.1016/j.etap.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima M, Fukami T, Yamanaka H, Higashi E, Sakai H, Yoshida R, et al. Comprehensive evaluation of variability in nicotine metabolism and CYP2A6 polymorphic alleles in four ethnic populations. Clin Pharmacol Ther. 2006;80:282–297. doi: 10.1016/j.clpt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Vogel RI, Carmella SG, Stepanov I, Hatsukami DK, Hecht SS. The ratio of a urinary tobacco-specific lung carcinogen metabolite to cotinine is significantly higher in passive than in active smokers. Biomarkers. 2011;16:491–497. doi: 10.3109/1354750X.2011.598565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, David SP, Tyndale RF, Wang H, Yu XQ, Chen W, et al. Relationship between amounts of daily cigarette consumption and abdominal obesity moderated by CYP2A6 genotypes in Chinese male current smokers. Ann Behav Med. 2011;43:253–261. doi: 10.1007/s12160-011-9318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao Y, Hoffmann E, Zia M, Bodin L, Zeman M, Sellers EM, et al. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 2000;58:747–755. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- 18.Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- 19.Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P., 3rd Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010;19:1160–1166. doi: 10.1158/1055-9965.EPI-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]