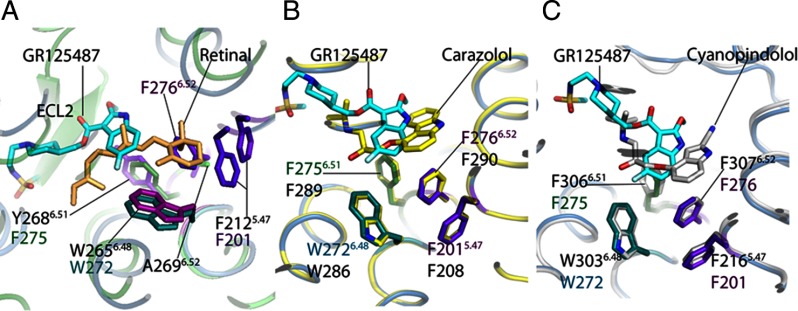
Fig. 3.
Comparison of the ligand-binding sites in GPCRs. A comparative analysis of docked antagonist GR125487 within the 5-HT4R model to crystal structures of 11-cis-retinal bound to rhodopsin (1F88) (A), carazolol bound to β2-AR (2RH1) (B), and cyanopindolol bound to β1-AR (C). The proposed toggle switch mechanism of rhodopsin activation can be seen in A. The β-ionone ring of retinal in the 11-cis-conformation (light pink) is sandwiched between Y2686.51 and F2125.47 (purple residues). The flip of the β-ionone ring forming all-trans-retinal allows swinging-in of W2656.48 to permit signaling in rhodopsin. B, comparison of the 5-HT4R-GR125487 model with the β2-AR·carazolol complex. The toggle switch mechanism of β2-AR is represented by four residues as follows: F2906.52 sandwiched between F2896.51, F2085.47, and W2866.48. Activation is proposed to trigger a rotation of W2866.48 to displace F2906.52. In 5-HT4R, residues F2756.51, F2766.52, F2015.47, and W2726.48 are conserved and positioned similarly around GR125487. C, comparison of binding of cyanopindolol to β1-AR with docked superposed GR125487. The four residues corresponding to the toggle switch are conserved in β1-AR, including F3066.51, F3076.52, F2165.47, and W3036.48. The length and branching of the tail group of GR125487 make its orientation different from those of the tail groups of carazolol and cyanopindolol. The green cartoon trace represents rhodopsin bound to 11-cis-retinal; the yellow trace is the β2-AR-T4L chimera bound to carazolol; the sea blue trace is the 5-HT4R model bound to GR125487, and the silver gray trace is the β1-AR bound to cyanopindolol.