Fig. 4.
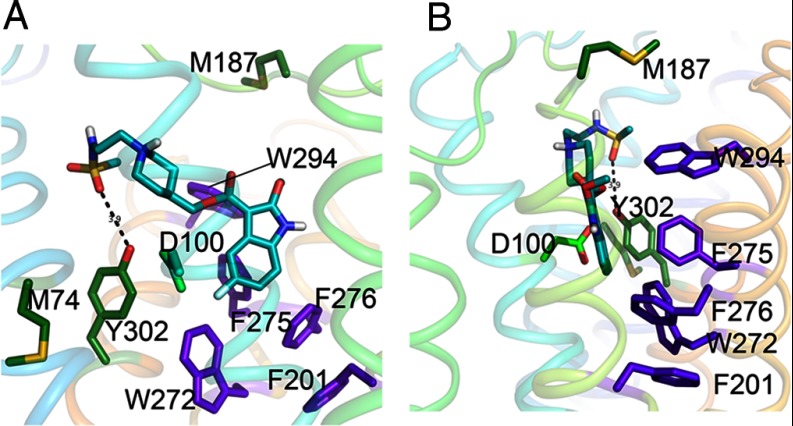
GR125487 interactions with hydrophobic residues in the binding site of 5-HT4R. A and B are views of binding pocket with a rotation of 90° along the vertical axis. For 5-HT4R, our model posits ring-face aromatic interactions of binding site residues that translate from the back face of the hetero-aromatic ring structure of GR125487 through a series of aligned aromatic residues. Apart from the highly conserved alignment of the toggle switch residues, a key difference in the binding site interactions for GR125487 in 5-HT4R involves W2947.35 on the back face of the heteroaromatic ring. The bulky aromatic ring of W294 interacts strongly with the heteroaromatic moiety of GR125487 through F2756.51 (purple), which continues down to F2766.52 (purple) and F2015.47 (purple), which in turn interact with W2726.48 (purple) and continues to Y3027.43 (green) on the front face. These strong ring-face aromatic hydrophobic interactions suppress toggle switch activation as long as the heteroaromatic headgroup of GR125487 remains bound in the 5-HT4R pocket to Y3027.43. Residues M742.58 and Y3027.43 are highlighted (forest green). Both of these residues are significantly protected upon antagonist binding, supporting their proximity to the binding site. This change is in contrast to M184, which shows no change in oxidation upon ligand binding. D1003.32 a key binding site residue, binds to 5-HT (serotonin) but not the antagonist (cyan). In our model of the ligand free state (not shown) D1003.32 is hydrogen bonded to Y3027.43.