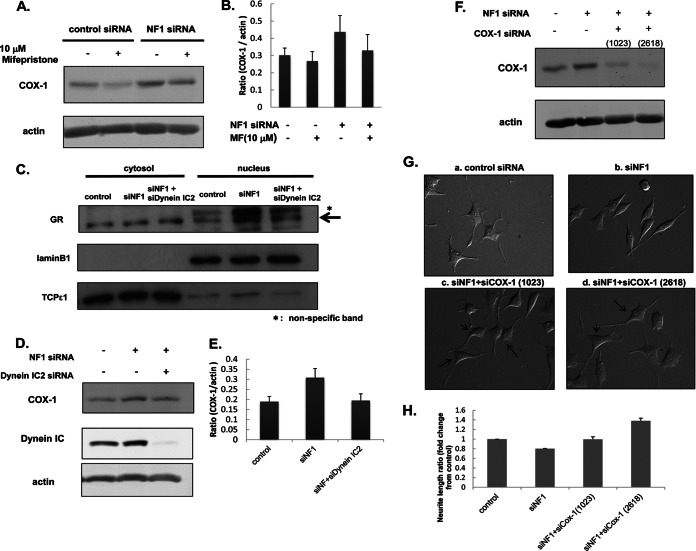
Fig. 6.
Biological validation of the abnormal dynein IC2-GR-COX1 network in NF1-KD PC12 cells identified by integrated proteomics. A and B, Up-regulation of COX-1 is suppressed by GR antagonist mifepristone. After 24-h NF1 or control siRNA transfection, PC12 cells were treated with 10 μm mifepristone for 30 min before treatment with NGF. After 48-h NGF treatment, cells were harvested for measurements of COX1 by immunoblot analysis. A, representative immunoblot image of COX1. Actin was used as the internal loading control. B, Quantification of COX1 expression. The normalized intensities of COX1 obtained from three separate identical experiments are shown in the histogram. Error bars represent S.E. of each sets of three experiments. C, Nuclear translocation of GR was decreased by the knockdown of dynein IC2. PC12 cells were transfected for 24 h with control siRNA, NF1 (249) siRNA, both NF1 (249) and dynein IC2 siRNAs before treatment with NGF. After 48-h NGF treatment, cells were harvested, and the cytoplasmic and nuclear proteins were extracted. Both cytoplasmic and nuclear fractions were subjected to immunoblot analysis using anti-GR antibody. TCPε1 and Lamin B1 were used as markers of the cytoplasmic and nuclear fraction, respectively. Representative images of three reproducible experiments are shown. D and E, COX-1 expression is down-regulated by dynein IC2 siRNA treatment. PC12 cells were transfected for 24 h with control siRNA, NF1 (249) siRNA, or both NF1 (249) and dynein IC siRNAs before treatment with NGF. After 48-h NGF treatment, COX-1 expression in the cells was analyzed by immunoblotting using anti-COX-1 antibody. D, Representative immunoblot images of COX1. Actin was used as the internal loading control. Representative images of three reproducible experiments are shown. E, Quantification of COX-1 expression. The normalized COX-1 intensities obtained from three separate identical experiments are shown in the histogram. Error bars represent S.E. of each set of three experiments. F, G, and H, COX-1 knockdown recovered the inhibition of neurite outgrowth in NF1-KD PC12 cells. PC12 cells were transfected for 24 h with control siRNA, NF1 (249) siRNA, both NF1 (249) and COX-1 (1023) siRNAs, or both NF1 (249) and COX-1 (2618) siRNAs before treatment with NGF. After 48-h NGF treatment, COX-1 expression in cells was analyzed by immunoblotting with anti-COX-1 antibody, and the neurite length were measured. F, Representative immunoblot images of COX-1. Actin was used as the internal loading control. G, Differential interference contrast images of PC12 cells treated with control siRNA [a], NF1 (249) siRNA [b], NF1 (249) and COX-1 (1023) siRNAs [c], and NF1 (249) and COX-1 (2618) siRNAs [d]. H, Measurement of neurite length of PC12 cells treated with the siRNAs. The average of the total length of PC12 neurites are shown on the y axis. The data are expressed as means and S.E. of three independent experiments. For each experiment, more than 50 cells were counted.