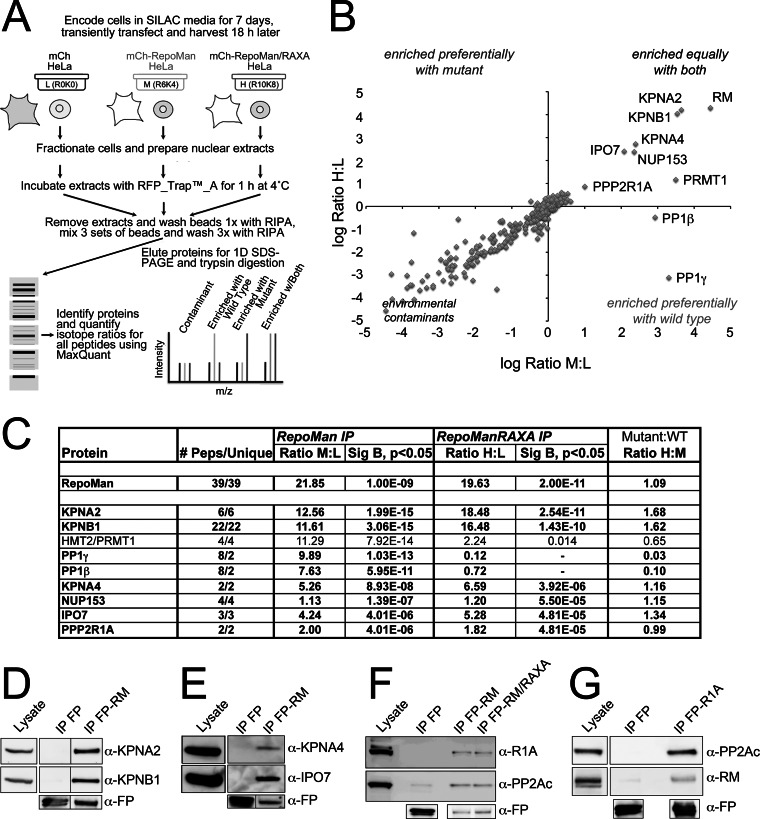
Fig. 1.
Quantitative analysis of the tagged RepoMan interactome. A, Design of the quantitative IP experiment comparing mCh-RepoMan (M), the non-PP1 binding mCh-RepoMan/RAXA mutant (H), and the mCh tag alone (L). B, Plot of log H:L ratio (enrichment in mCh-RM/RAXA IP) versus log M/L ratio (enrichment in mCh-RM IP). Contaminants cluster around a log ratio 0 (SILAC ratio 1:1), whereas proteins enriched equally with both fall in the upper right quadrant. C, Table listing # peptides (total and unique), ratio M/L (enrichment with mCh-RepoMan), ratio H/L (enrichment with mCh-RepoMan/RAXA) and ratio H/M (enrichment with mutant relative to wild type) for proteins with M/L ratios > 2 identified with two or more peptides. Significance B (Sig B) values, which are weighted by relative intensity, are shown for those proteins with ratios significantly different from the mean (p < 0.05). Proteins highlighted in bold have been validated as bona fide RepoMan interactors by IP/WB analysis. D, Validation of the copurification of KPNB1 and KPNA2 with tagged, full-length RepoMan (RM) from asynchronous cell extracts. E, Co-IP/WB validation of novel interactions of RepoMan with two additional importins, KPNA4 and IPO7. F, Copurification of PPP2R1A with FP-tagged wild type and RAXA mutant RepoMan. G, Reciprocal validation of the R1A/RepoMan interaction demonstrated by copurification of endogenous RepoMan with FP-tagged R1A. As a control, PP2Ac was also shown to copurify with FP-R1A. Anti-FP antibodies demonstrate the amount of fusion protein (or free FP) recovered in each IP.