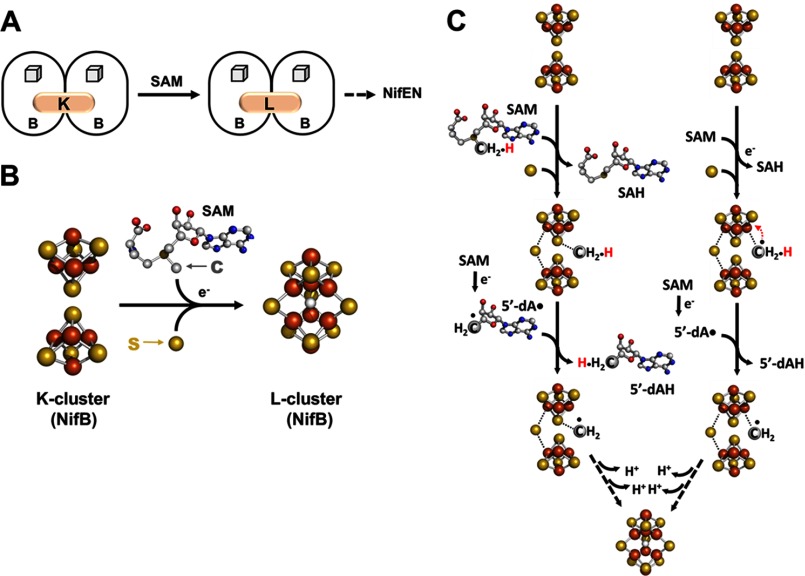
FIGURE 2.
Formation of an 8Fe core. A, NifB catalyzes the SAM-dependent conversion of an [Fe4S4] cluster pair (the K-cluster) to an [Fe8S9] cluster (the L-cluster), which is subsequently delivered to NifEN. The SAM motif-associated [Fe4S4] clusters on NifB are depicted as cubes, whereas the transient clusters on this protein are represented by pink ovals and are labeled accordingly. B, structural details of the K-cluster (left) and L-cluster (right) on NifB. The clusters are shown as ball-and-stick models, with the atoms colored as follows: iron, orange; sulfur, yellow; oxygen, red; carbon, gray; and nitrogen, dark blue. PyMOL was used to create this figure (Protein Data Bank code 3PDI). C, two proposed mechanisms of carbon insertion by NifB. One involves the transfer of a methyl group via an SN2 mechanism, followed by the formation of a methylene radical upon hydrogen atom abstraction by 5′-dA• and the subsequent transfer of this radical intermediate to a sulfur atom of the K-cluster (left), whereas the other involves the formation of a methyl radical via reductive cleavage of SAM, followed by the transfer of this transient intermediate to an iron atom of the K-cluster and the subsequent processing of this intermediate into a methylene radical (right). SAH, S-adenosyl-l-homocysteine.