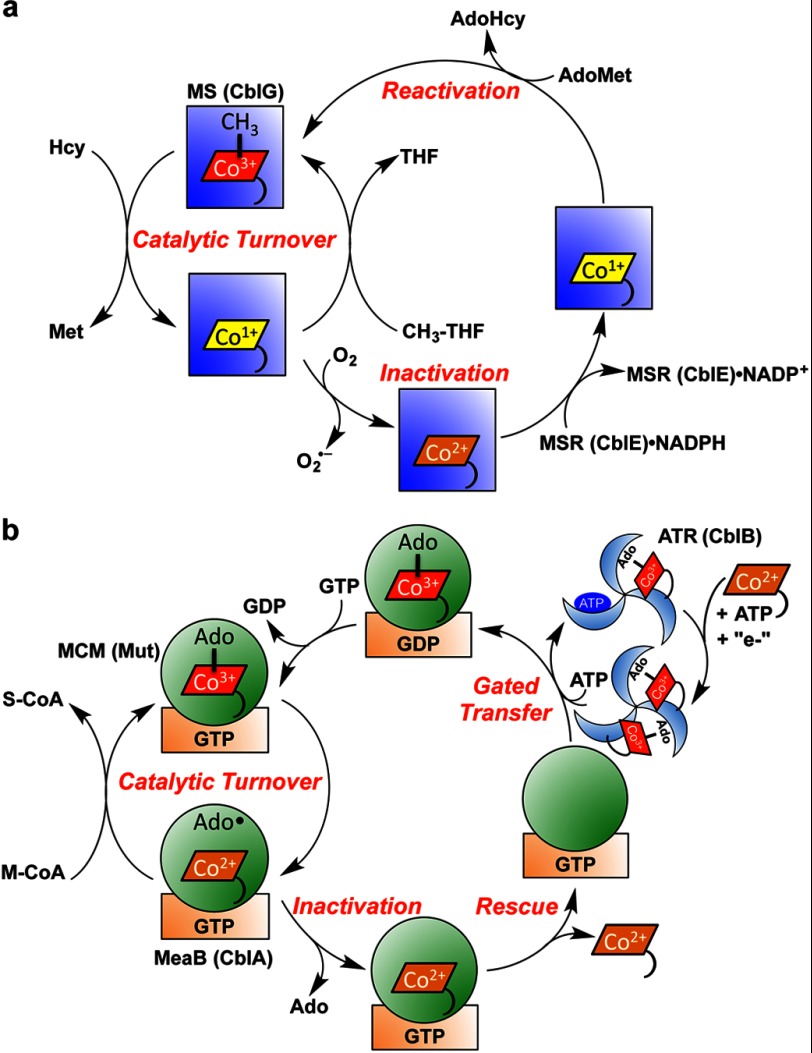
FIGURE 4.
Cofactor loading, activity, and repair of mammalian cobalamin-dependent enzymes. a, methionine synthase (MS; CblG; blue square) catalyzes the overall transfer of a methyl group from N5-methyltetrahydrofolate to homocysteine to give methionine and tetrahydrofolate (THF). Occasional oxidative escape of the cob(I)alamin intermediate during the catalytic cycle leads to the inactive cob(II)alamin species. The latter is rescued to MeCbl in a reductive methylation reaction needing NADPH, methionine synthase reductase (MSR; CblE), and AdoMet. This repair reaction is also likely to represent the route for formation of MeCbl following transfer of cob(II)alamin to apomethionine synthase. The mechanism for cob(II)alamin transfer during methionine synthase reconstitution is not known. AdoHyc, S-adenosylhomocysteine. b, ATR (CblB; blue wheel) converts ATP and cob(II)alamin in the presence of a reductant to AdoCbl. Two equivalents of AdoCbl are bound at one time, and binding of ATP to the vacant site triggers transfer of one AdoCbl to the MCM (Mut; green circle) active site in a reaction that is gated by GTP hydrolysis in the G-protein chaperone (MeaB or CblA; orange rectangle). The GTP-bound state of MeaB blocks transfer of cob(II)alamin from ATR to the complex between MCM and G-protein. During catalysis, the cobalt-carbon bond is cleaved homolytically to initiate a radical-based mechanism for the conversion of methylmalonyl-CoA (M-CoA) to succinyl-CoA (S-CoA). Occasional loss of 5′-deoxyadenosine (Ado) from the active site precludes re-formation of AdoCbl at the end of the catalytic cycle and leads to inactive mutase. In this situation, the GTP-containing chaperone promotes dissociation of cob(II)alamin, permitting reconstitution of the mutase with active cofactor.