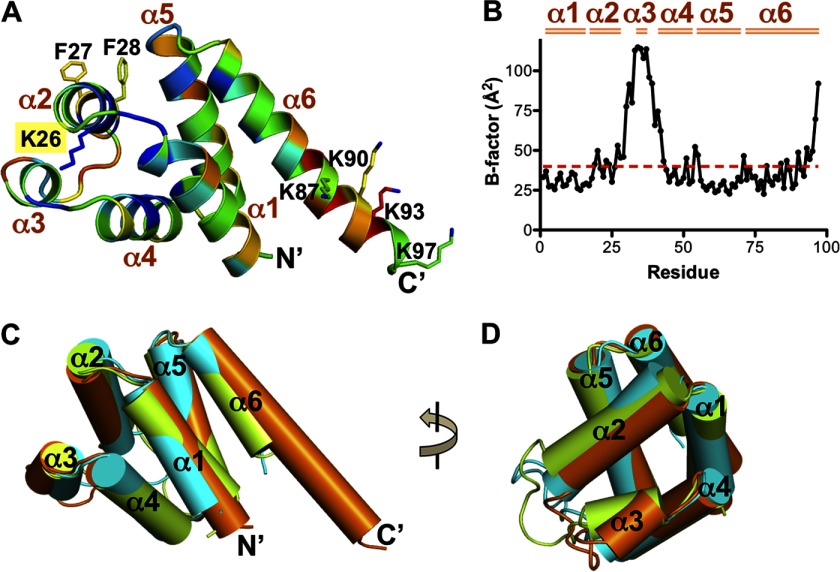
FIGURE 2.
Overall structure of the AIM PYD. A, ribbon diagram of the AIM2 PYD structure colored based on sequence conservation scores calculated by the ConSurf server (47). The color is from blue (conserved) to red (non-conserved). Residues Lys-26, Phe-27, and Phe-28 at the α2 helix and Lys-87, Lys-90, Lys-93, and Lys-97 at the α6 helix are shown as ball-and-stick models. The six α helices and both termini are labeled. B, a plot of the temperature factors for the AIM2 PYD residues. The average temperature factor of the domain is indicated as a red dotted line, and the six α helices are marked above the plot. C, superposition of the PYD structures from AIM2 (orange), ASC (green), and NLRP3 (cyan) with each helix represented as a cylinder. D, a 90° rotation of the view in C.