FIGURE 1.
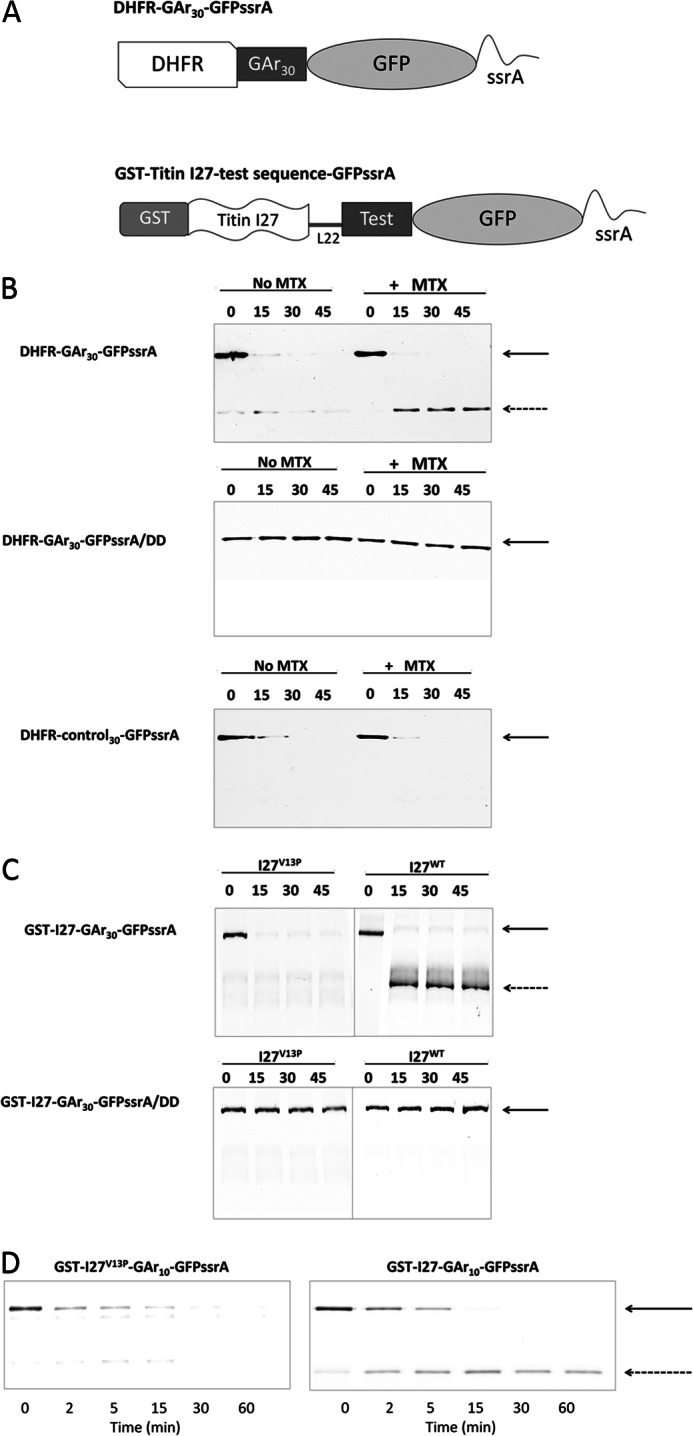
The accumulation of degradation intermediates requires collaboration between a GAr and a stable folded domain. A, schematic representation of substrate proteins used in this paper. A human DHFR or titin I27 immunoglobulin coding sequence was included to provide a domain requiring active unfolding by the ClpX translocase/unfoldase. Sequences of varying length and amino acid composition (Test), some of which consist of Gly-Ala repeat sequences (GAr) were placed between DHFR or I27 and GFP-ssrA to determine their effects on the production of intermediate products of stalled degradation. L22, a linker of 22 amino acids. B, top, degradation of DHFR-GAr30-GFP-ssrA in the presence (+MTX) or absence (No MTX) of 20 μm methotrexate. Bands corresponding to full-length parent substrate (solid arrow) or degradation intermediates (dashed arrow) were detected by Western blotting using anti-DHFR. Middle, as in the top, but the substrate contains a terminal dipeptide mutation of ssrA that cannot be recognized by ClpX. Bottom, as in the top, but DHFR-control30-GFP-ssrA contains a 30-mer diverse amino acid sequence instead of GAr30. C, top, I27V13P-GAr30-GFP-ssrA and I27-GAr30-GFP-ssrA substrates were subjected to degradation by ClpXP. Bottom, the same proteins (GST-I27-GAr30-GFP-ssrA/DD) but with a terminal dipeptide mutation of ssrA that cannot be recognized by ClpX. D, degradation of GST-I27-GAr10-GFP-ssrA. The titin I27 domain contains a destabilizing V13P point mutation (left) or is wild type (right).
