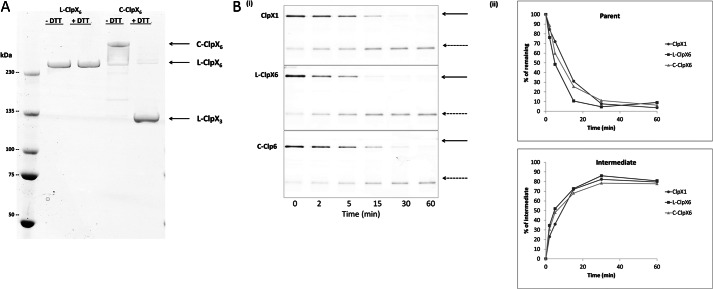
FIGURE 7.
Degradation rate and intermediate production by various topological forms of ClpX. A, molecular mobility of ClpX covalent linear (L-ClpX6) and closed circular (C-ClpX6) hexamers. Proteins were fractionated by SDS-PAGE in the absence of dithiothreitol (−DTT) or the presence of dithiothreitol (+DTT), conditions that maintain or reduce disulfide bonds, respectively. L-ClpX6 and C-ClpX6 were generated and purified as described under “Experimental Procedures.” C-ClpX6 is a dimer of linear covalent trimers, in which the two trimer junctions are connected by disulfide bonds. The C-ClpX6 protein consists of ∼85% closed circular hexamer (two disulfide bonds) and ∼15% linear hexamer (one disulfide bond). Disulfide bonds in C-ClpX6 were broken in the presence of 10 mm DTT; thus, the ClpX trimer (ClpX3) was the sole species present under +DTT reducing conditions. B, monomeric ClpX (ClpX1), L-ClpX6, and C-ClpX6 were compared in assays of I27-GAr10-GFP-ssrA degradation. i, time-dependent substrate degradation and production of intermediates. ii, quantitation of data in i. Data were generated and analyzed as in Fig. 2.