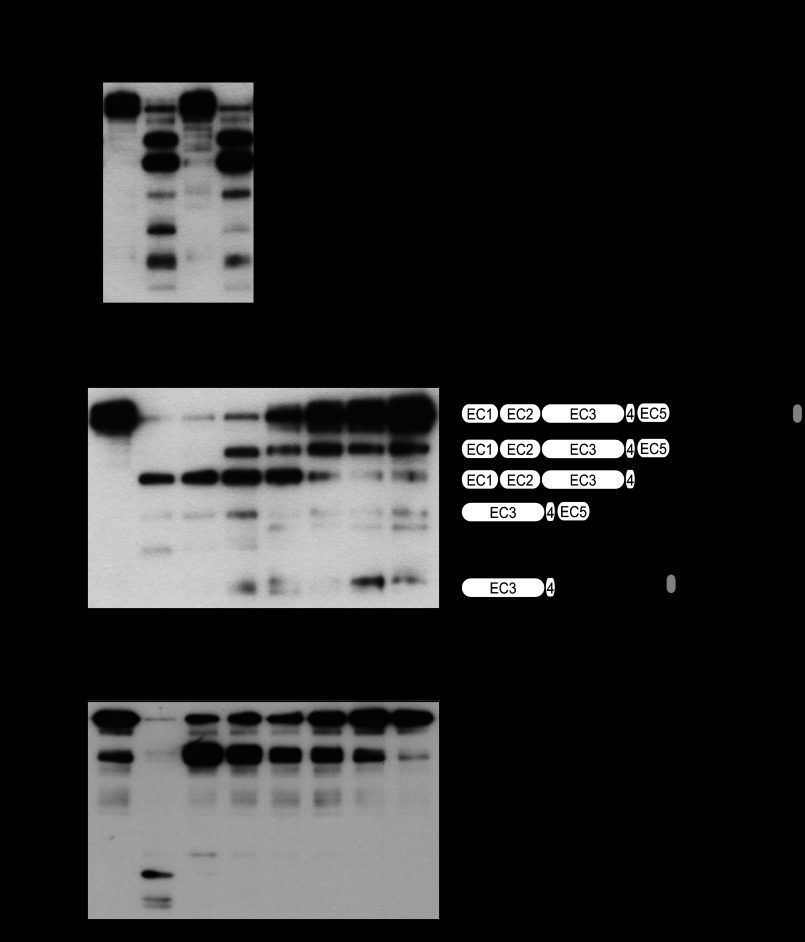
FIGURE 3.
SpeB cleaves intercellular junctional proteins. A, recombinant E-cadherin was treated with culture supernatants from strain NIH35, an speB deletion mutant, and a complemented strain for 6 h at 37 °C. Cleavage of E-cadherin was detected by Western blotting. B, SpeB activities present in the culture supernatants from several GAS clinical isolates were analyzed using FITC-labeled casein. For quantitative evaluation, the casein hydrolysis activity of recombinant SpeB was used to prepare a standard curve. To ensure the specificity of SpeB, the cysteine protease inhibitor E-64 was routinely added to selected samples. All of the experiments were performed six times with three technical repeats. The data are shown as the means ± S.D. of six samples from representative findings obtained in three independent experiments. C, recombinant E-cadherin was incubated with various concentrations of recombinant SpeB for 6 h at 37 °C. Cleavage of E-cadherin was detected by Western blotting. Five N-terminal residues in the cleavage fragments of E-cadherin were determined by peptide sequence analysis. The scheme represents estimated cleavage fragments of E-cadherin. D, full-length recombinant occludin was incubated with various concentrations of recombinant SpeB for 6 h at 37 °C.