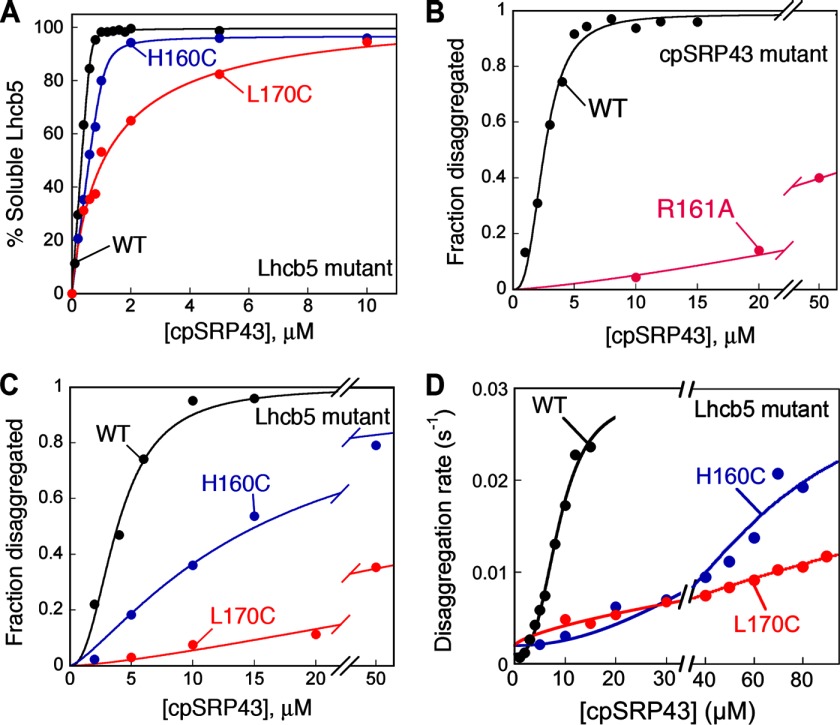
FIGURE 4.
L18-binding mutants uncouple initial recognition of the aggregate from its subsequent solubilization. A, binding of cpSRP43 to wild-type Lhcb5 and L18 mutants H160C and H170C. The data were fit to Equation 1 (see “Experimental Procedures”) and gave Kdapp values of 10 nm for wild-type Lhcb5 (black), 30 nm for Lhcb5-H160C (blue), and 1.1 μm for Lhcb5-L160C (red). B, concentration dependences for the equilibrium of disaggregation of LHCP by wild-type cpSRP43 (black) or mutant cpSRP43(R161A) (magenta). C and D, chaperone concentration dependences for the equilibrium (C) and kinetics (D) of disaggregation of Lhcb5 (black), Lhcb5-H160C (blue), and Lhcb5-L170C (red) by wild-type cpSRP43.