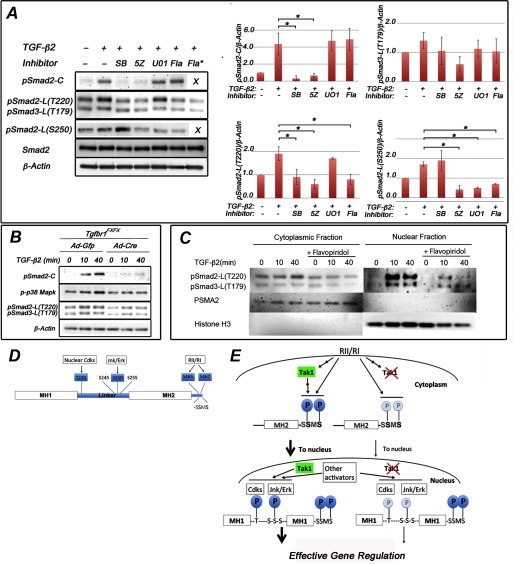
FIGURE 5.
Smad2 linker-region at Thr-220 is phosphorylated by nuclear kinases in palatal mesenchymal cells. A, representative immunoblot of primary palatal mesenchymal cells stimulated with TGF-β2 in the presence of SB431542 (SB), 5Z-7-oxozeaenol (5Z), UO126 (UO1), and flavopiridol (Fla; 1 and 10 μm (asterisk)). Protein lysates were analyzed for Smad2 C-terminal phosphorylation, Smad2 linker region phosphorylation (at Thr-220 and Ser-250), and Smad3 linker region phosphorylation (at Thr-179). The bar graphs show relative quantification of pSmad2-C, pSmad-L(T220), pSmad3-L(T179), and pSmad2-L(S250) (normalized to β-actin; n = 3). Error bars, S.E. *, p < 0.05. B, representative immunoblot of primary palatal mesenchymal cells from Tgfbr1FX/FX embryos were transduced with Ad-Gfp or Ad-Cre, the cells were stimulated with TGF-β2, and protein lysates were analyzed for C-terminal Smad2 phosphorylation, p38 Mapk phosphorylation, and linker region phosphorylation at Smad2-L(T220) and at Smad3-L(T179). β-actin was loading control. C, palatal mesenchymal cells were stimulated by TGF-β2 in the presence and absence of flavopiridol. Cytosolic and nuclear lysates were analyzed for Smad2 linker region phosphorylation at Thr-220 (pSmad2-L(T220)) and for Smad3 linker region phosphorylation at Thr-179 (pSmad3-L(T179)). Antibodies against PSMA2 and histone H3 were used to assess the purity of cytosolic and nuclear fractions (middle and bottom panels). D, schematic representation of Smad2 depicting Thr/Ser residues phosphorylated by nuclear Cdks and Jnk/Erk in the linker region and by TGFβRI (RI) at the C terminus examined in this study. E, schematic summary illustrating the role(s) of Tak1 in phosphorylation of Smad2 (left side of diagram) in which Smad2 C-terminal phosphorylation levels were greatly enhanced in response to TGF-β stimulation when Tak1 was present, as well as Tgfbr1. Cdk-dependent phosphorylation of Thr-220 occurs in the nucleus. Phosphorylation of Ser-250 is Mapk-dependent but also inhibited by Cdk inhibitor, which may be due to some dependence on the prior phosphorylation of Thr-220 in the model of Aragón et al. (9). Deletion of Tak1 results in reduced C-terminal phosphorylation, leading to a reduced nuclear accumulation of Smad2, and reduced linker region phosphorylation (right side of diagram), which would be expected to reduce effectiveness in gene regulation according to the model of Aragón et al. (9).