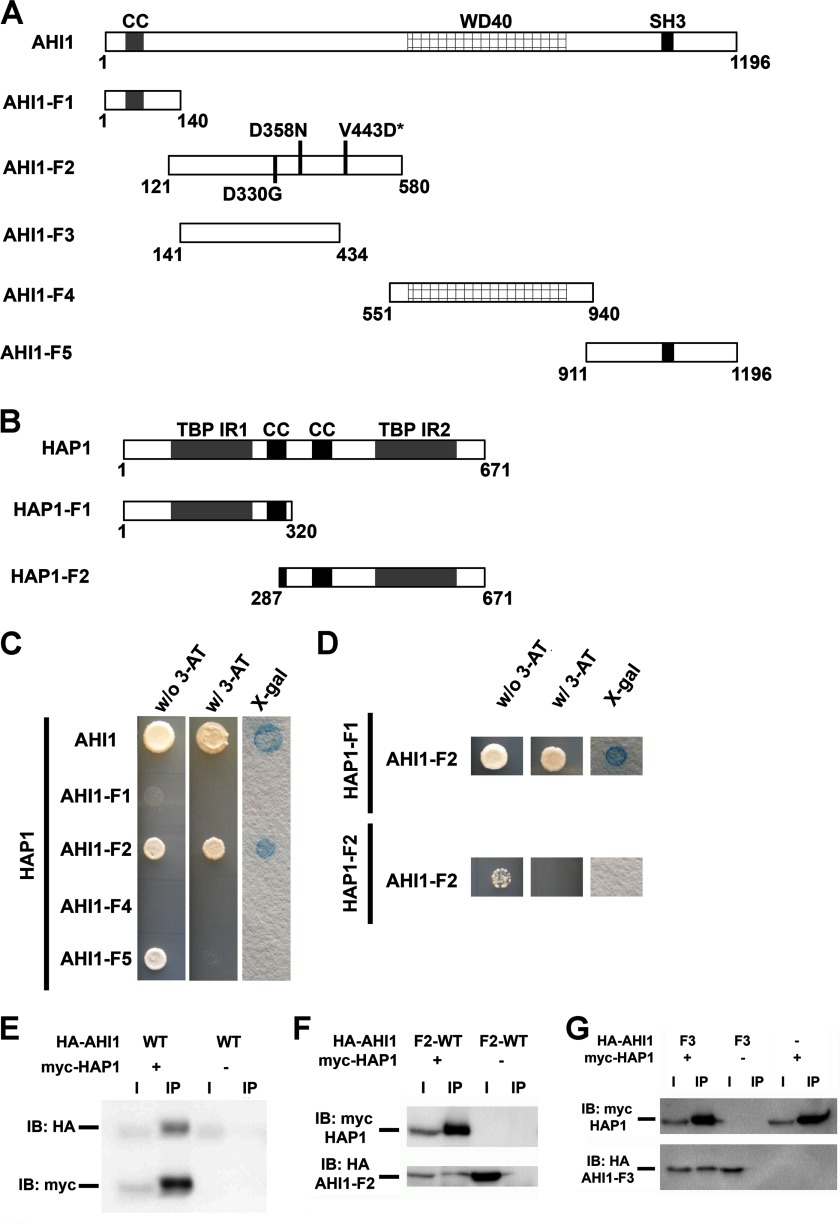
FIGURE 7.
Human AHI1 and HAP1 directly interact. A, diagrams representing full-length AHI1 and five fragments of AHI1 for assaying its binding to HAP1. B, diagram representing full-length HAP1 and two HAP1 fragments that were used to determine the AHI1 binding region. C, full-length AHI1 and full-length HAP1 directly interact as assessed by directed yeast two-hybrid analyses. The coiled-coil domain (AHI1-F1), the WD40 repeat domain (AHI1-F4), and the SH3 domain (AHI1-F5) of AHI1 did not exhibit any direct interactions with HAP1. However, a region of AHI1 between the coiled-coil domain and the WD40 repeats (AHI1-F2) that appeared to not have any definitive protein motif does demonstrate a yeast two-hybrid interaction with HAP1. D, directed yeast two-hybrid assays showed that only HAP1-F1, which contains the N-terminal region of HAP1, exhibited a positive binding with AHI1-F2 as demonstrated by a positive result in an X-Gal filter lift-off assay. E, positive interaction of full-length AHI1 and HAP1 was demonstrated in co-immunoprecipitation assays from lysates of HEK293T cells transiently co-expressing myc-HAP1 and HA-tagged full-length AHI1. Myc antibodies were used for the immunoprecipitation. F, AHI1-F2 binds HAP1 by co-immunoprecipitation with myc. G, a smaller fragment of AHI1-F2, AHI1-F3, still binds HAP1 by co-immunoprecipitation with myc. CC, coiled-coil domain; SH3, SH3 binding motif; TBP-IR, TATA-binding protein interacting region; IB, immunoblot.