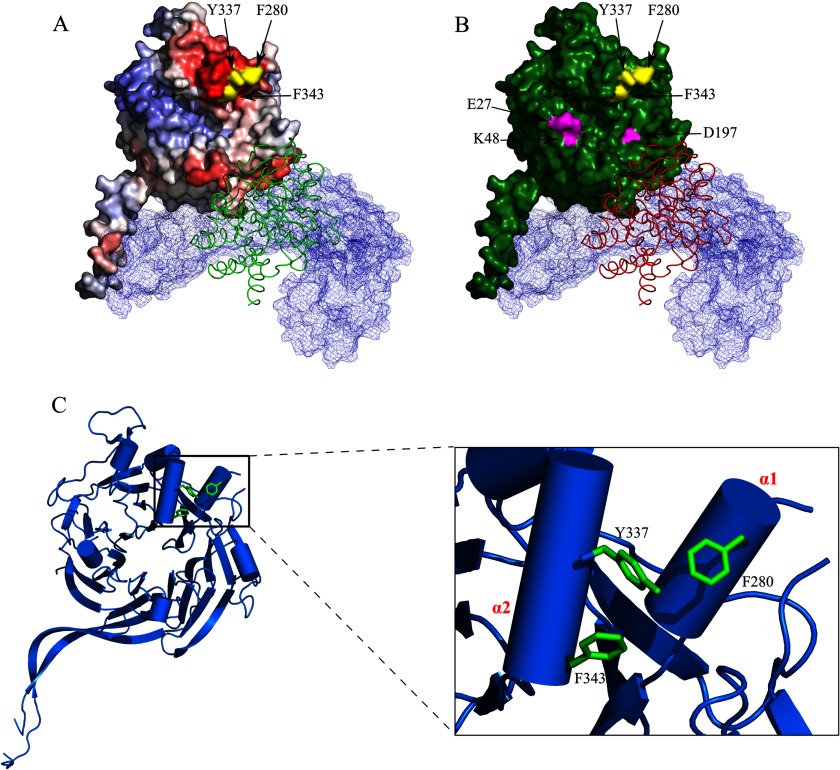
FIGURE 7.
The E4orf4 binding site in PP2A-B55α. A, the location of the B55α residues Phe-280, Tyr-337, and Phe-343 required for E4orf4 binding is shown. The B55α subunit is colored according to the Promate protein-protein interaction prediction as described in Fig. 1B, and the B55α residues Phe-280, Tyr-337, and Phe-343 are colored in yellow. The PP2A A subunit is mesh-surfaced in light blue, and the ribbon representing the C subunit is colored in green. B, the E4orf4 binding site in B55α does not overlap with the Tau binding site. B55α residues Phe-280, Tyr-337, and Phe-343 are colored in yellow, and the Tau protein binding residues Glu-27, Lys-49, and Asp-197 (28) are colored in magenta. The B55α subunit is colored in green. The PP2A A and C subunits are represented as described in A, except that the C subunit is colored red. C, the B55α residues Phe-280, Tyr-337, and Phe-343 are part of the α1 and α2 helices and face each other. The B55α structure including the core β-propeller (ribbons) and the α-helices (cylinders) is shown. Residues Phe-280, Tyr-337, and Phe-343 are colored in green. A close-up of the α1,α2 helices is shown on the right, displaying residues Phe-280, Tyr-337, and Phe-343 as they protrude from the α1 and α2 helices, facing each other.