FIGURE 1.
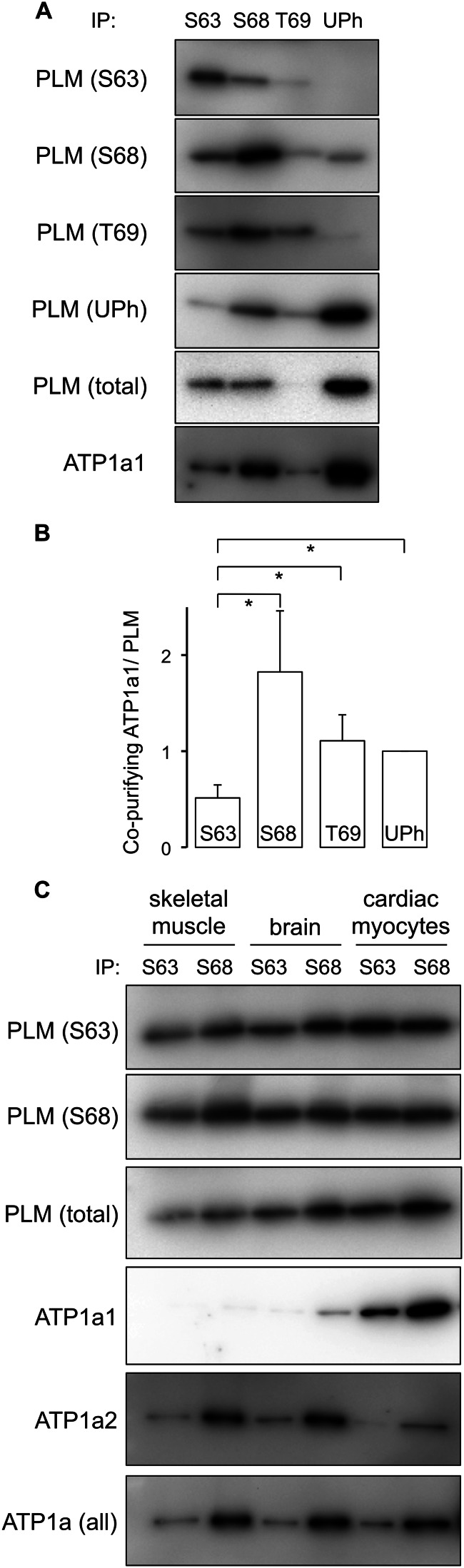
PLM phosphorylated at Ser63 co-immunoprecipitates poorly with the Na pump α subunit. A, PLM was immunoprecipitated (IP) from ARVMs using antibodies specific for Ser63 (S63), Ser68 (S68), and Thr69 (T69) phosphorylated and unphosphorylated (UPh) forms. In the representative immunoblots shown, although the PLM-Ser63 antibody precipitates a substantial amount of PLM, co-precipitation of sodium pump α1 subunit is relatively poor, particularly compared with the adjacent Ser68 immunoprecipitation, which precipitates the same amount of PLM but a considerably larger amount of sodium pump α1 subunit. B, quantitative analysis of Western blot data presented in A. For comparison purposes, the quantity of sodium pump α1 subunit co-precipitated by each antibody was normalized to the quantity of PLM precipitated in the same reaction. To allow comparison of multiple experiments, this ratio was arbitrarily defined as equal to 1 for the immunoprecipitation reactions using antibodies specific for the unphosphorylated form of PLM. *, p < 0.05 (n = 7). C, co-immunoprecipitation reactions from homogenates of whole brain, skeletal muscle (quadriceps), and ARVMs using antibodies specific for PLM phosphorylated at Ser63 and Ser68. Na pump α subunits from both brain and skeletal muscle exhibit the same preferential enrichment of the pump α subunit in PLM-Ser68 immunoprecipitation reactions only.