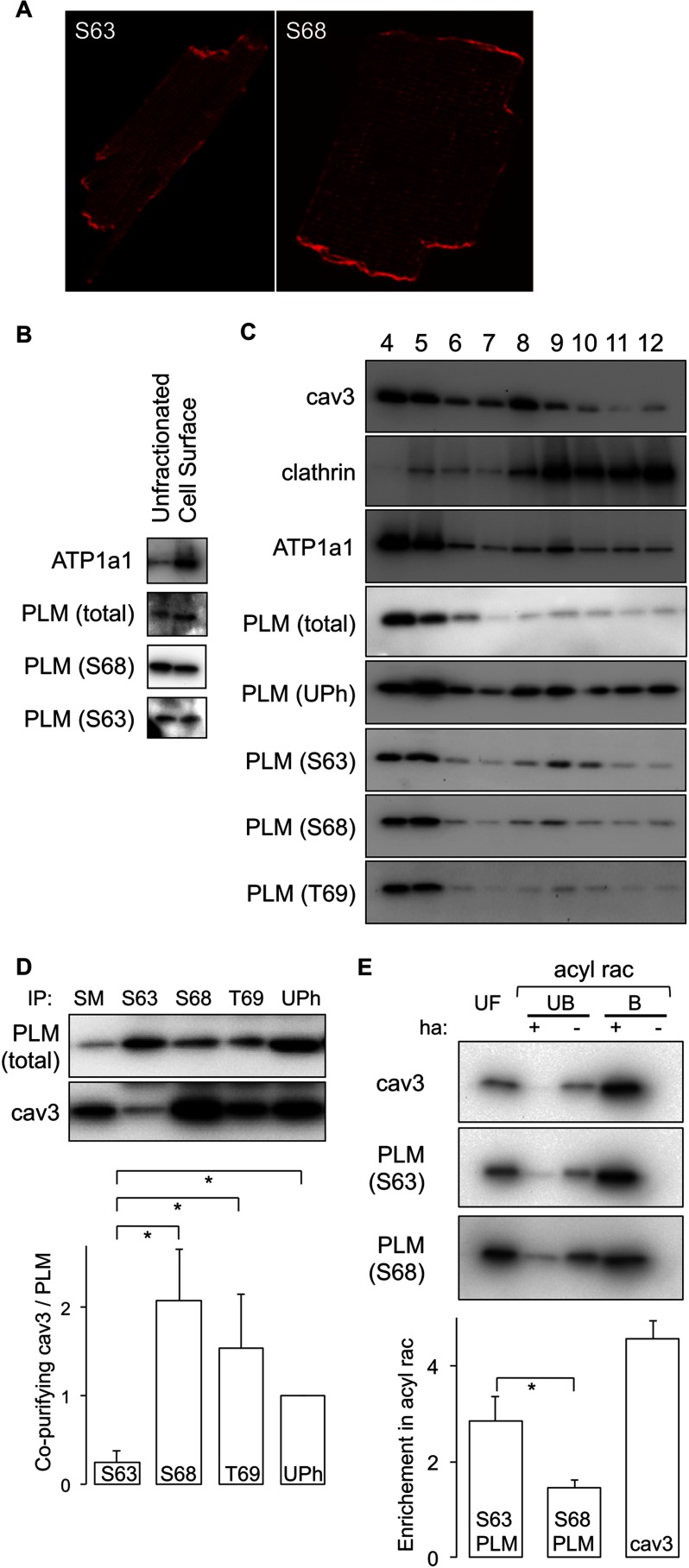
FIGURE 3.
Subcellular localization of Ser63-phosphorylated PLM. A, immunofluorescent localization of Ser63 (S63) and Ser68 (S68) phosphorylated PLM in ARVMs. Both forms are distributed grossly identically in myocyte surface membranes in t-tubules, surface sarcolemma, and intercalated disks. B, cell surface fractions were prepared from ARVMs using cell-impermeable biotinylation reagents and analyzed alongside the unfractionated cell lysate from which they were derived. Ser63- and Ser68-phosphorylated forms are equally enriched in cell surface membranes compared with unfractionated starting material. C, sucrose gradient fractionation of ARVMs to purify a caveolar/lipid raft component. All phosphorylation states and unphosphorylated (UPh) PLM are localized in buoyant membranes from sucrose gradients (fractions 4 and 5), which are also enriched in the caveolar marker protein caveolin 3 (cav3) but not the bulk sarcolemma marker clathrin. T69, Thr69. D, phosphospecific immunoprecipitations (IP) of PLM indicate very poor co-purification of caveolin 3 with Ser63-phosphorylated PLM despite its localization to the caveolar membrane compartment. Quantitative analysis of the relative co-purification of caveolin 3 with PLM in phosphospecific co-immunoprecipitation reactions is shown (for an explanation of the calculation see legend to Fig. 1). SM, immunoprecipitation starting material. *, p < 0.05 (n = 7). E, palmitoylated proteins were purified by acyl rac in the presence (+) and absence (−) of hydroxylamine. UF lanes, unfractionated starting material; UB lanes, proteins not purified by acyl rac; B lanes, proteins purified by acyl rac. Ser63-phosphorylated PLM is purified to a considerably greater extent relative to the unfractionated starting material than Ser68-phosphorylated PLM, indicating that it is considerably more palmitoylated. *, p < 0.05 (n = 5).