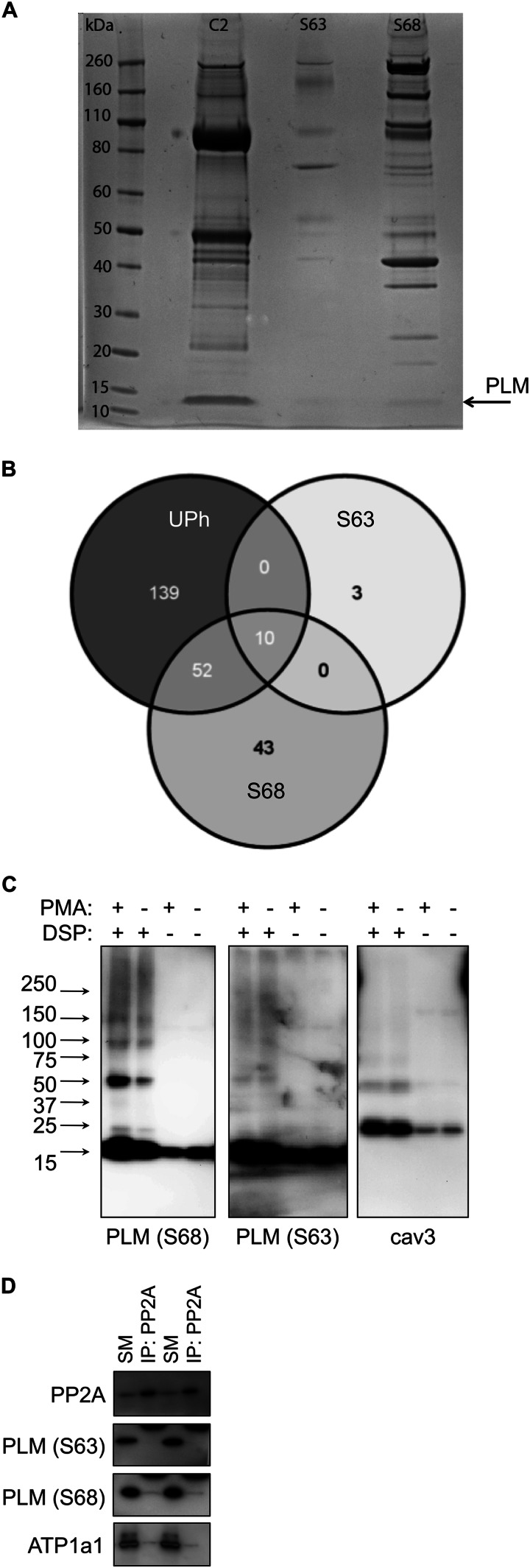
FIGURE 4.
Protein interactions of Ser63-phosphorylated PLM. A, co-immunoprecipitation reactions using antibodies specific for unphosphorylated (C2), Ser63-phosphorylated (S63), and Ser68-phosphorylated (S68) PLM. Despite purifying similar quantities of PLM, the number and abundance of co-purifying proteins are substantially lower in the Ser63 immunoprecipitation reaction compared with the Ser68 immunoprecipitation reaction. B, co-immunoprecipitation reactions shown in A were analyzed by LC-MS/MS to identify all proteins. Venn diagram showing overlap in the proteins identified from each reaction. Protein identifications are shown in Table 1 and supplemental Table S1. UPh, unphosphorylated. C, control and PMA-treated ARVMs (300 nm, 10 min) were treated with the homobifunctional cross-linker DSP (1 mm, 60 min at 4 °C) and immunoblotted as indicated. No unique interactions for Ser63-phosphorylated PLM were revealed. cav3, caveolin 3. D, PP2A catalytic subunit was immunoprecipitated from ARVMs, and co-purifying sodium pump α1 and PLM phosphorylation states were immunoblotted. Ser63-phosphorylated PLM does not co-purify with PP2A. Duplicate immunoprecipitations are shown. SM, immunoprecipitation starting material; IP, immunoprecipitation.