Table 2.
Enantioselective Synthesis of Chromenyl Pivalate from Phenols
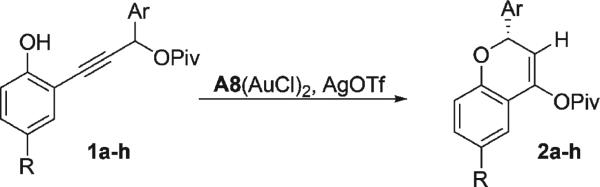
| |||||
|---|---|---|---|---|---|
| entrya,b | 1 | R | Ar | % yieldc | % eed |
| 1 | 1a | H | Ph | 85 | 91 |
| 2 | 1b | H | p-BrC6H4 | 86 | 86 |
| 3 | 1c | H | 2-Naph | 68 | 84 |
| 4 | 1d | H | o-MeC6H4 | 77 | 84 |
| 5 | 1e | H | p-iPrC6H4 | 73 | 89 |
| 6 | 1f | H | p-CF3C6H4 | 76 | 88 |
| 7 | 1g | Me | Ph | 85 | 90 |
| 8 | 1h | Br | Ph | 83 | 83 |
Conditions: CDCl3 (0.1 M), 5 mol % A8 · (AuCl)2, 10 mol % AgOTf for 4 h at 0 °C.
Absolute stereochemistry assigned by analogy to 4e (Table 3).
Isolated yield of product after column chromatography.
Determined by chiral HPLC.
