Table 3.
Enantioselective Synthesis of Chromenyl Pivalate from Phenol Ethers
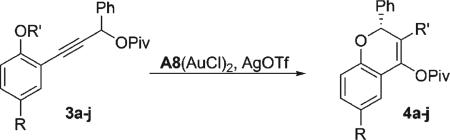
| |||||
|---|---|---|---|---|---|
| entrya,b | 3 | R | R' | % yieldb | % eed |
| 1 | 3a | H |
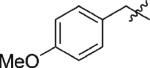
|
92 | >99 |
| 2 | 3b | Me | 75 | >99 | |
| 3c | 3c | Me |

|
62 | 99 |
| 4 | 3d | H |
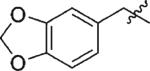
|
91 | >99 |
| 5 | 3e | H |
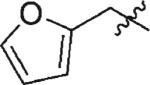
|
59 | 99 |
| 6c | 3f | H |

|
75 | >99 |
| 7 | 3g | H |
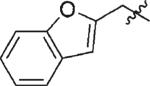
|
75 | 97 |
| 8 | 3h | H |
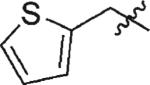
|
73 | 99 |
| 9c | 3i | H |

|
73 | 96 |
| 10 | 3j | H |
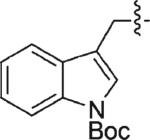
|
94 | 95 |
Conditions: CDCl3 (0.1 M), 5 mol % A8 · (AuCl)2, 10 mol % AgOTf for 4 h at 0 °C.
Isolated yield of product after column chromatography.
At room temperature.
Determined by chiral HPLC.
