Abstract
The selection of foods to eat is a complex interplay of vision, taste, smell, and texture. In addition to micro- and macronutrients, plant-based foods also contain several classes of phytochemicals. In many cases, the phytochemicals account for the various colors of foods. Although aesthetically pleasing, the color of foods may mislead consumers as to their phytochemical content, which is particularly true with regard to polyphenols. Polyphenols are a broad class of compounds with antioxidant and other health benefits. Human vision is limited to a small window (390–765 nm) of the electromagnetic spectrum. Many important phytochemicals (e.g., vitamin C) have no absorbance in this range. Therefore, the human eye cannot directly judge the vitamin C content of foods. Being able to see in the ultraviolet range allows bees to locate the pollen-rich region of flowers, whereas pit vipers locate their prey by being able to “see” them in the infrared range. Assessing the impact of phytochemicals on human health depends on several factors. Colorless phytochemicals in unprocessed foods may be lost during the cooking process because no visual guide exists to ensure their retention. The molecular structures of phytochemicals influence the extent to which they are altered by cooking processes and the methods by which they are absorbed from the gastrointestinal tract. Extensive metabolism by phase I/II enzymes and by the gut microbiome may also create compounds that the eye is never allowed to appreciate.
Introduction
Knowledge concerning which foods are suitable for eating was gained by trial and error over tens of thousands of years. With the introduction of agricultural practices, food availability became more certain. Before the arrival of national and international travel, food choices were determined by the plants and animals that could be domesticated within the region, local environmental conditions that affect crop growth (altitude, latitude, temperature, and available water supply), seasonal changes, food storage capabilities between seasons (and sometimes years), and the nature of the foods themselves (i.e., taste, texture, and smell). Through modern interstate and international travel, a wide variety of foods is now available to almost all societies, providing a far greater choice of foods than ever before.
In addition to macronutrients (carbohydrates, fats, proteins, and fiber) and micronutrients (vitamins and minerals), all plant-based foods contain phytochemicals that form the nonnutritive part of the diet. These secondary metabolites are produced for the benefit of the plant rather than for the consumer. However, these phytochemicals may also affect the health of the consumer. Beneficial effects may be derived from carotenoids, flavonoids, and other phytochemicals. Consuming whole foods with their associated phytochemicals may provide greater health benefits due to synergistic effects between the phytochemicals (1). However, phytochemicals also include alkaloids and polyketides with undefined or even toxic effects, and this factor must be considered when making food choices.
Role of color in foods
Some researchers have emphasized the role of color in the optimal selection of foods (2). Color can improve the aesthetics of foods, and consuming colorful foods is considered the essence of good eating in some cultures. However, color is not necessarily an accurate guide concerning the presence of beneficial or toxic phytochemicals within foods.
Senses that control appreciation of foods
Although the electromagnetic spectrum spans wavelengths from gravity waves to gamma rays, the human eye is limited to a narrow range (390–765 nm) (3). Humans can hear sounds (20Hz–20 kHz) and feel heat (infrared range = 1–10 μm), but our visual range is very small. What we see depends on specific chemical regions within phytochemicals. Vitamin C is colorless to the human eye because it does not absorb light in the visual range. Instead, vitamin C absorbs light in the ultraviolet (UV) range (245–265 nm) (4) and in the infrared range (3, 6, and 7.5 μm) (5). Humans only began to recognize vitamin C–containing foods by their effects in reversing scurvy on long sea voyages (6), which was well before the invention of spectrophotometric methods.
In contrast to humans, pit vipers (e.g., rattlesnakes and copperheads) have a heat-sensing pit organ that is located between the eye and nostril on each side of the face (7). The pit organ is sensitive to the infrared heat emitted by warm-blooded prey and allows the snake to detect its prey in the dark. The night vision equipment used extensively by the US Armed Forces uses a similar principle. The infrared color (temperature) of the prey then changes after it has been bitten and killed.
Taste
The sense of taste is governed by cells located on the tongue as well as within the gastrointestinal tract that contain taste receptors, particularly G-protein–coupled receptors. These receptors recognize sweet, bitter, salty, sour, and umami tastes. Humans have many more taste receptors than dogs (8). In contrast to humans, cats cannot detect “sweetness” due to the loss of the taste receptor type 1 member 2, which has a microdeletion (−247 bp) that leads to a premature stop codon (9). Differences in detecting individual flavors may account for eating behaviors in both people and other animals.
Smell
Almost 900 genes form the olfactory receptor family in humans, but many of these are pseudogenes and may not be expressed (10). Mice and other mammals have even more olfactory genes (11). Humans have a sophisticated system for detecting different smells, but the human olfactory system is far less sensitive than that found in certain animals. Bloodhounds have 10–100 million times the sensitivity to smells compared with humans (12).
Physical events that cause human eyes and retinas to detect color
Photon level
Color in the visible range (390–765 nm) results from photons causing transitions of electrons from a ground state to a quantized excited state (Fig. 1). Photons that correspond to the particular energy transition are absorbed by the compound. The broad spectrum of white light is then depleted of these photons, and the light that reaches the eye appears to be “colored.” The excited electrons do not remain in the excited state for very long and disperse their excess energy via vibrational energy states before emitting photons. However, these emitted photons are at longer wavelengths than the incident photons because they have lower energies. If the wavelength of the emitted photons is >765 nm, the human eye cannot see them at all. If the compound is excited by UV light, the emitted photons may be in the visual range, and fluorescence is observed. Fluorescence occurs on a very rapid timescale (ns) and depends on constant excitation by incident light. In contrast, phosphorescence occurs because of the much longer lifetime of a modified (triplet) excited state. Phosphorescent light emission does not require continuous incident light and can also be caused by chemical, nonphoton excitation of electrons.
Figure 1.
Diagram of ground and excited states of electrons. Incident photons of the right energy can cause electrons in their ground state to jump to permitted excited states. In excited states, some of this energy can be transferred to vibrational states, causing electrons to decay to lower energy states. Photons emitted during the energy transfer process as electrons decay to their original ground state are of lower energy (and hence longer wavelength) than the incident light. If the emitted light is in the visual range, fluorescence is observed.
Eye level
The sense of color depends on the response of cells in the retina and the ability of the brain to process the information it receives via the optic nerve. Light entering the eye is controlled by the iris, which is similar to an aperture on a camera. The iris consists of muscles that narrow or widen the pupil of the eye in response to the intensity of incoming light. Bright, intense light narrows the pupil, whereas low light in the evening or at night causes the iris to maximize pupil size.
The light then passes through the lens, which focuses the light on the retina. The optical density of the lens varies with wavelength, resulting in almost full transmission at longer wavelengths (>650 nm). Only a small percentage of incident light passes through the lens at 400 nm (13). Therefore, what we see is a biased representation of what really occurs. Cataractous processes in the lens that occur with aging reduce the transmission and increase the scattering of light and can also result in blindness (13).
The rods and cones in the retina contain photoreceptors. Rod cells are far more numerous than cone cells and can detect light under low-light conditions, sometimes even single photons. However, rod cells do not discriminate color because they contain only 1 light-sensitive pigment (rhodopsin), which has its greatest sensitivity to green-blue light (498 nm) (14). Rhodopsin is also insensitive to wavelengths >650 nm. The low light levels in some restaurants may serve to reduce the ability of diners to determine the visual quality of their food.
Color is detected by the less numerous cone cells, which have 3 different light-sensitive pigments (14). Cone cells are most sensitive to light at 420 nm (blue light), 534 nm (green light), and 564 nm (red light). Humans are trichromatic, as are apes, Old World monkeys, and birds (15). Many New World monkeys are dichromatic, usually having only blue and green photoreceptors, and cannot differentiate between green and red colors. Bees have photoreceptors that detect UV light, which allow them to locate the pollen-rich region of flowers (Fig. 2) (16). Some women appear to be tetrachromatic (17). Because photoreceptor genes are located on the X chromosome, only women can have 2 copies of a photoreceptor gene. A mutation of one of the copies may lead to a shift in optimum wavelength sensitivity, providing these women with an increased ability to distinguish colors.
Figure 2.

Two perspectives of a dandelion. The familiar yellow color of a dandelion as seen by humans is shown on the left. The dandelion on the right is seen by bees that can detect light in the UV range. Reproduced with permission from Bjorn Rorslett and the Science Photo Library (B539/0972 and B539/0973).
Most signals from rods and cones pass down the optic nerve to the visual cortex. The visual cortex is where vision is interpreted (18). Some of the axons of the optic nerve end in the pretectal nucleus (reflexive response coordination), whereas others end at the suprachiasmatic nucleus (sleep-wake cycle regulation). The information that passes down the ganglions in optic nerve fibers is chemical (not spectral) information and is not colored. This information represents the degree to which each 1 of 3 types of cone photoreceptors can detect light. Blue photoreceptors respond to light in the 400–540 nm range, with detection peaking at 454 nm. Green photoreceptors respond to light in the 400–660 nm range, with detection peaking at 534 nm. Red photoreceptors respond to light in the 420–700 nm range, with detection peaking at 564 nm. For an object emitting, reflecting, or transmitting light at 450 or 500 nm, all 3 types of photoreceptors contribute to the signal. At 450 nm, signals from blue photoreceptors are dominant. At 500 nm, the signals are approximately equal for each type of photoreceptor. Green and red photoreceptors dominate at 540 and ⩾600 nm, respectively.
Object level
The color of an object depends on how it reflects or absorbs incoming light. If all light is reflected, the object appears white (or whatever the incoming light happens to be). The object appears black if it absorbs all incoming light at visual wavelengths. Light reflected without being scattered produces a mirror effect. Some foods display a “sheen” or “gloss,” which is a form of (partial) reflection. Clear gels have a low light-scattering effect, so light passes through them to an observer. Gels can also selectively absorb light at particular wavelengths, causing them to appear colored.
Color from phytochemicals and flavonoids
Three major classes of phytochemicals exist in colored foods: carotenoids (yellow to red), chlorophylls (green), and anthocyanins (blue, purple, and red). Anthocyanins are a particular type of flavonoid within the polyphenol family.
Carotenoids
The conjugated dienes in carotenoids provide their color (β-carotene = orange, lycopene = red, lutein = yellow) (Fig. 3). The number of conjugated dienes within a carotenoid influences the level of electron delocalization that can occur. A greater number of conjugated dienes increases the red appearance of a carotenoid by causing a greater depletion of light at the shorter wavelengths. Carotenoids are produced in chloroplasts and chromoplasts, both of which are involved in photosynthesis. Carotenoids are physiologically important to humans, but humans are incapable of producing these compounds. Therefore, carotenoids must be consumed in the diet.
Figure 3.
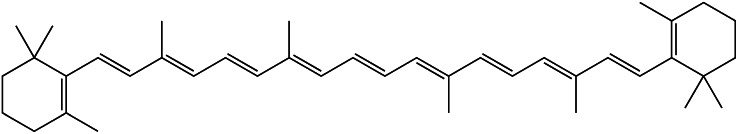
Chemical structure of β-carotene. β-Carotene is a carotenoid pigment synthesized by plants whose orange color is caused by conjugated unsaturated bonds.
Carotenoids are cleaved to form retinol and then transported to the eye. In the eye, retinol is converted to retinal, an essential factor for vision. Retinal binds to photoreceptors and, when struck by a photon, undergoes isomerization of its 11-cis double bond to the all-trans state, which transmits a signal. Although foods containing carotenoids are associated with better health (19), supplementing the diet with carotenoids has not provided the expected positive health benefits (20). Carotenoids can capture lipid free radicals, especially light-induced singlet oxygen, thereby acting as antioxidants. In this form, however, carotenoids also become oxidants. Larger doses of carotenoids in supplementation studies produced significant concentrations in the vascular system with the potential for causing oxidative damage.
Chlorophyll
The green color of chlorophyll is caused by a magnesium atom complexed to a chlorin center (Fig. 4), which is similar to hemoglobin in that iron binds to a porphyrin center to produce a red color. Chlorophyll is vital for the photosynthesis that takes place in plant chloroplasts and algae (21). Because chlorophyll absorbs light in the blue and red, but not in the green, range of the visual spectrum, it appears green. Chlorophyll a and chlorophyll b are the 2 principal chlorophylls, and they absorb light in the red range (680 and 700 nm) of the visual spectrum. Light causes chlorophyll to release an electron to form chlorophyll+. The reduction in chlorophyll+ results in the oxidation of water to O2, which is critical for the development (and sustenance) of oxygen-breathing life on the planet.
Figure 4.
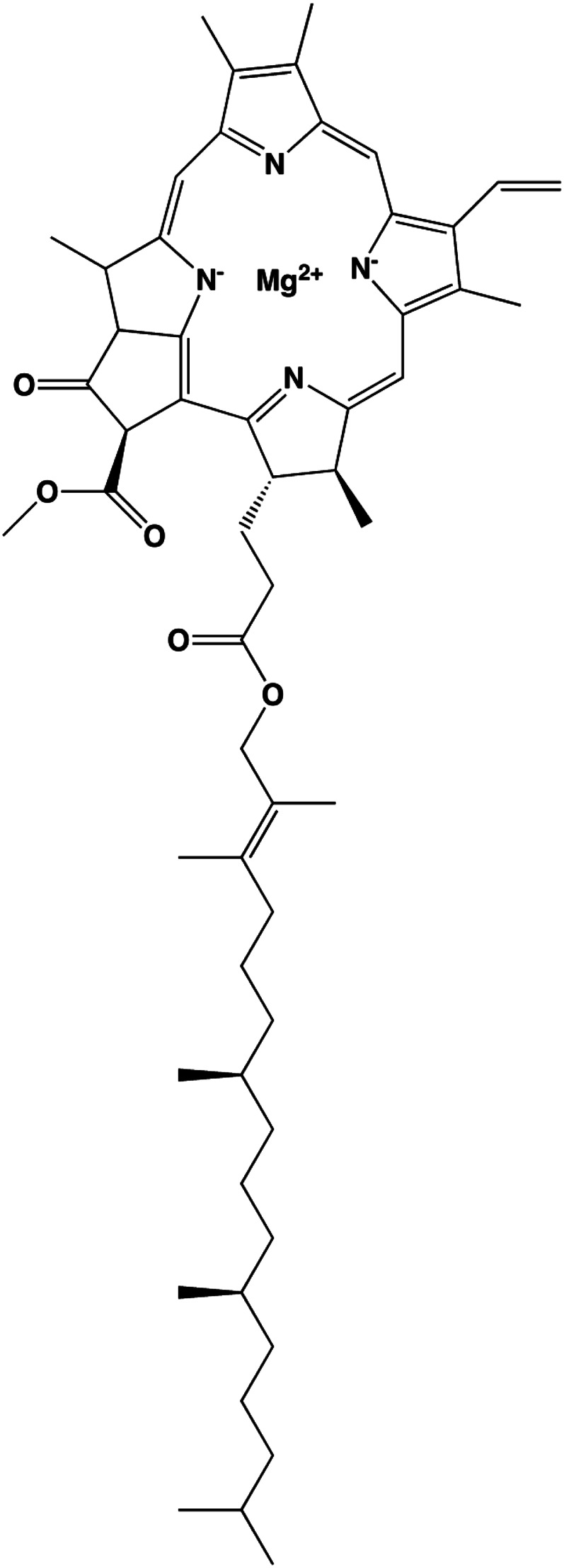
Chemical structure of chlorophyll a. Chlorophyll a is one of the major green pigments of plants.
Anthocyanins
A positively charged oxygen atom in the heterocyclic ring produces the color for anthocyanins (Fig. 5). This group of flavonoids is sensitive to changes in pH. At a low (acidic) pH, they appear pink or white, but then change to blue or purple at a neutral pH. The blue-purple color results from their absorption in the green range of the visual spectrum (∼500–520 nm). Anthocyanins whose absorption extends into the blue region will appear red. Anthocyanins are glycosides of anthocyanidins and are present in high concentrations in many edible berries (22) and in the purple forms of otherwise white vegetables (23).
Figure 5.
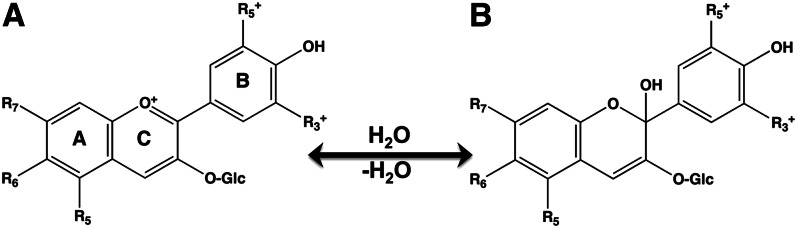
Chemical structures of anthocyanins. Anthocyanins appear pink at an acidic pH (A), but appear red-purple-blue as the pH moves towards neutrality (pH 7) (B). In fruit and vegetables, anthocyanins are typically conjugated to glycosides. Different anthocyanins have a variety of substituents [R] in positions 5 through 7 in the A ring and in positions 3* and 5* in the B ring.
Other flavonoids
The remaining flavonoids are not colored. These flavonoids principally absorb wavelengths in the UV (240–330 nm) and infrared (>1 μm) ranges (5). Overall, color is a poor guide for gauging the flavonoid and other phytochemical content of foods and should not be used as the sole basis for choosing which fruits and vegetables to consume. Visual cues overemphasize the importance of foods containing colored flavonoids versus noncolored flavonoids.
Flavonoids include several subclasses (flavones, flavonols, flavanones, and flavanols) that are based on substitutions in and saturation of their heterocyclic ring. In a minor group of flavonoids, the phenyl ring is shifted from the 2-position to the 3-position (isoflavonoids). Isoflavones are produced in tropical leguminous plants (24), such as the soybean (Glycine max) and the kudzu vine (Pueraria lobata).
Flavonoids in white vegetables
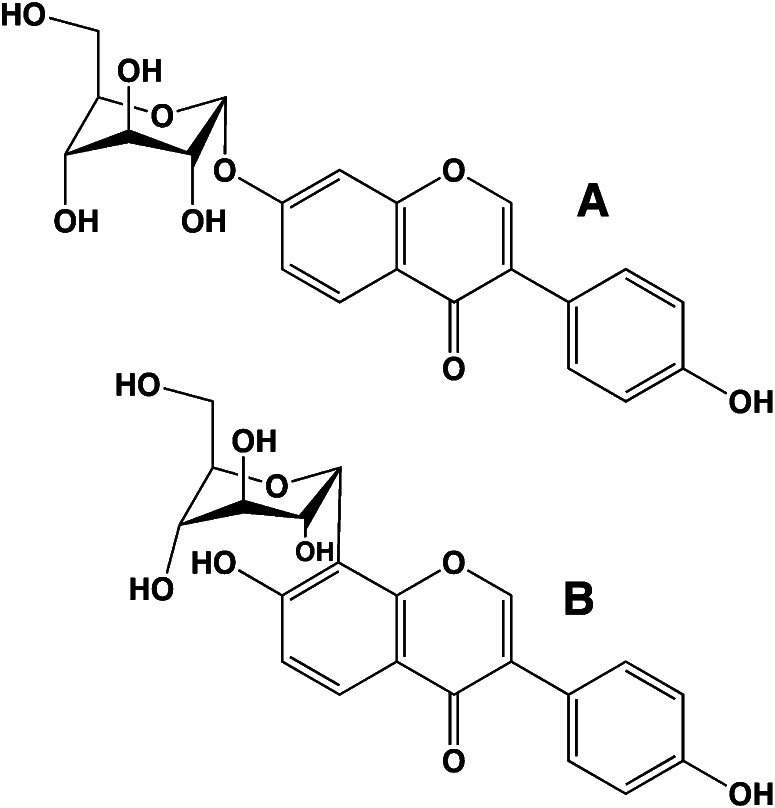
Vegetables vary widely in their flavonoid content. Even white or nearly white vegetables, such as potatoes, contain flavonoids. Of the common vegetables, soybeans have the highest isoflavone content (1–2 mg/g) (25, 26), but the American groundnut (Apios americana) is the vegetable with the highest isoflavone concentration (genistein glycosides at 8 mg/g) (27). The American groundnut is a tuber that is similar to the potato, but it has a much higher protein concentration. This tuber was being consumed by eastern Native Americans at the time of the first European settlers and has lately been of some interest to the USDA as a crop for the 21st century (28). Although modern biologists have engineered increased pathways of flavonoid biosynthesis, potatoes, particularly those with white flesh, have much lower levels of flavonoids (29). Potatoes from higher elevations in South America are often purple and may even be variegated (30). These potatoes contain anthocyanins that become blue after cooking. Concentrations of anthocyanins as high as 16 mg/g have been reported for these blue potatoes (31).
Potatoes and other “white” vegetables are generally eaten in large servings. Mean potato consumption in the United States is 100 g (0.2 pounds)/d (32), which provides 7, 3, 7, and 23% of the daily requirement for vitamins B-1 (thiamin), B-2 (riboflavin), B-3 (niacin), and B-6, respectively. Potatoes also provide 24 and 2% of the daily requirement for vitamins C and K, respectively. These values are based on consumption of whole potatoes (flesh and skin). The skin of the potato may also contain glycoalkaloids (e.g., solanine) (Fig. 6). Glycoalkaloids are toxins that can be found in potatoes and other members of the Solanum dulcamara (nightshade) family. In the potato, these toxins are typically present only at very low concentrations. Significant amounts are usually indicated by the appearance of a green color (chlorophyll) on the potato (33), which, in this case, is a warning for toxicity.
Figure 6.
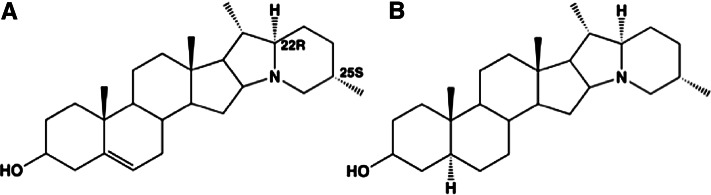
Chemical structures of colorless potato solanine alkaloids. Solanidine (A) and demissidine (dihydro form) (B) are shown conjugated to a glycoside (solatriose).
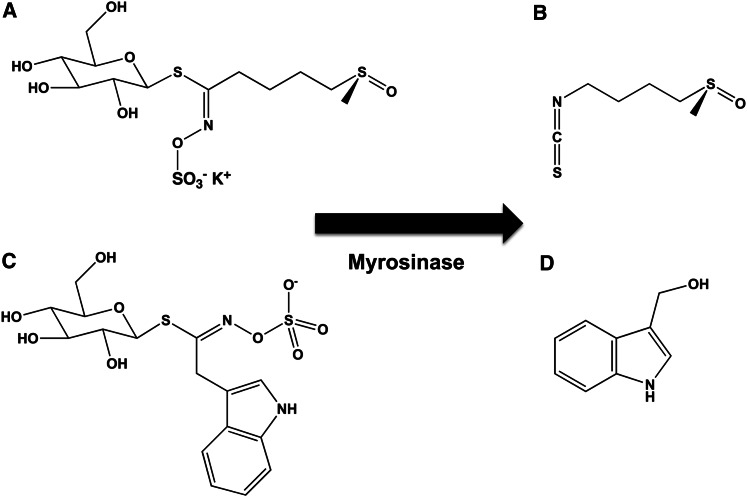
Although orange, green, and purple varieties occur, cauliflower is generally white. The orange variety has 25 times the amount of β-carotene than the others (34), and the purple variety contains anthocyanins. Cauliflower has other noncolored phytochemicals that have been associated with health benefits, including sulforaphane (Fig. 7A, B) and indole-3-carbinol (Fig. 7C, D). Sulforaphane is produced from glucosolinates via hydrolysis during cooking or chewing. Both of these compounds have shown anticancer properties in model systems (35–37).
Figure 7.
Chemical structures of sulforaphane and indole-3-carbinol. Sulforaphane (A, B) and indole-3-carbinol (C, D) are colorless chemopreventive agents that are formed from precursors by the enzyme myrosinase. Myrosinase and the required precursors are found in cruciferous vegetables (e.g., broccoli, cauliflower, and cabbage).
Chemistry and bioavailability of flavonoids
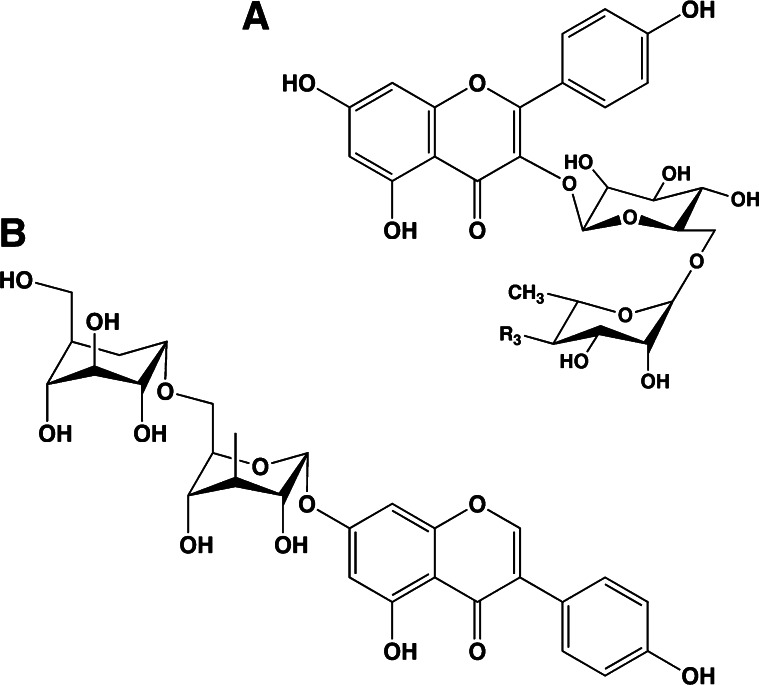
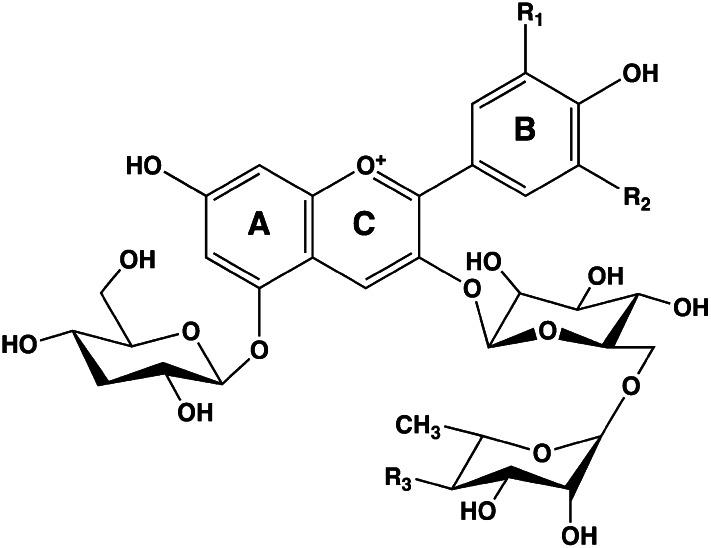
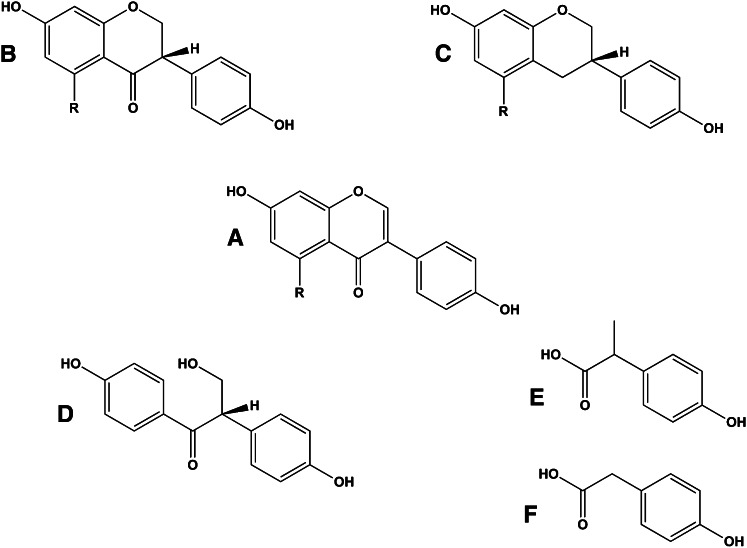
Flavonoids in plants are typically found as glycosides. Glycosides are molecules in which a sugar is bound to a noncarbohydrate functional group. Some flavonoids are conjugated with simple monosaccharides, such as glucose, rhamnose, and arabinose, which can be further esterified with malonic acid. Certain flavonoids have more complicated glycosides. The principal flavonoid in A. americana is 7-glucosylglucoside of genistein (Fig. 8A) (27), whereas the potato contains 3-triglucoside-7-rhamnoside of kaempferol (Fig. 8B) (38). Flavonoids can be located either in the flesh or the skin of a vegetable. Blue potatoes from the Andes contain complex glycosides of anthocyanins, including delphinidin, petunidin, cyaniding, and peonidin. The predominant anthocyanin common to several cultivars is petunidin-3-p-coumaroyl-rutinoside-5-glucoside (Fig. 9) (31).
Figure 8.
Chemical structures of glucosyl-7β-glucosylgenistein (A) and kaempferol 3-rutinoside (B). These colorless flavonoids are found in potatoes (kaempferol 3-rutinoside) and the tubers of Apios americana (glucosyl-7β-glucosylgenistein).
Figure 9.
Chemical structures of potato anthocyanin glycosides. These anthocyanins have been found in Andean blue potato species (31). In the principal anthocyanin, R1 = OCH3, R2 = OH, and R3 = p-coumaric acid. Other R3 substituents include caffeic and ferulic acids. Redrawn from reference 31.
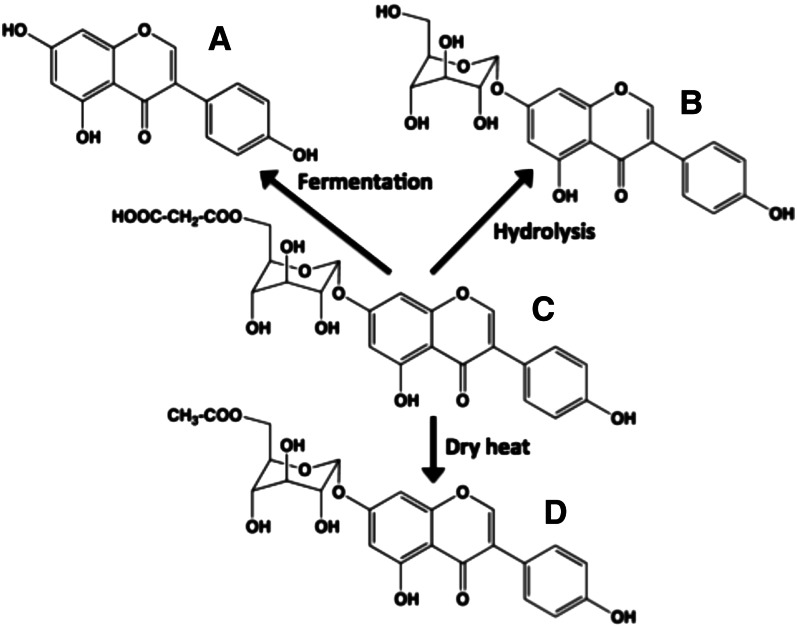
Different flavonoid chemistries can have profound effects on actual flavonoid intake. Flavonoids with polyglycosyl groups are far more water-soluble than other flavonoids. Therefore, vegetables that are roasted, baked, or eaten with skin may yield the highest flavonoid intakes. Boiling vegetables causes the loss of these types of flavonoid glycosides. Unless the flavonoid is highly hydroxylated (e.g., green tea catechins), the monoglycoside conjugates of flavonoids are much less water-soluble. Heating vegetables with superheated water can result in the loss of malonyl esters, whereas dry heat can cause decarboxylation (Fig. 10) (39). Because these flavonoids are colorless, the consumer would not realize the loss.
Figure 10.
Effects of cooking on glucosides of isoflavone genistein. The major isoflavonoid species in intact soybeans before processing are 6′-O-malonyl-β-glucoside conjugates. (C) 6′-malonyl-7β-glucosylgenistein undergoes 3 major chemical changes during processing: (A) fermentation to yield aglycone genistein, (B) hydrolysis of the malonyl group via superheated steam to yield genistin (7β-glucosylgenistein), and (D) decarboxylation of the malonyl group via dry heat to yield 6′-acetyl-7β-glucosylgenistein.
Systemic absorption of flavonoids varies widely (40). Many monoglycoside conjugates of flavonoids are hydrolyzed by an enzyme (lactose phloridzin hydrolase) in enterocytes located in the wall of the small intestine (41). Hydrolysis of these conjugates produces a sugar component and an aglycone (the flavonoid). Because aglycones appear in the blood within 30 min after consumption, these flavonoids appear to be absorbed well via passive diffusion in the small intestine (42) and the stomach. The absorption rate for aglycones is dependent on Lipinski’s “Rules of Five” established for pharmaceutical agents (43). The increased water solubility of polyhydroxylated flavonoids decreases their rate of passive absorption (39, 40). Diglycoside conjugates of flavonoids also have increased water solubility (27). Complex flavonoid glycosides that are esterified are poor substrates for lactose phloridzin hydrolase, and their hydrolysis is dependent on hydrolases contained in the bacteria of the large bowel (39). Therefore, their uptake is delayed for several hours after consumption (4–6 h) due to the increased transit time from the small intestine to the colon. In most cases, flavonoids passing through the enterocytes are reconjugated to form β-glucuronides and sulfate esters with only small amounts of aglycones entering the systemic circulation (39, 40).
In addition to hydrolysis and phase II conjugation, flavonoids undergo substantial metabolism by bacteria in the large bowel. These reactions involve reduction in flavanones, flavans, and ring-opened metabolites (Fig. 11) (39, 40). These metabolites can be absorbed, conjugated in the liver, and secreted into bile. Many are readily excreted in urine. The extent of metabolism by colonic bacteria varies from individual to individual. Equol, a bacterial metabolite of the isoflavone daidzein, is typically found in ∼30% of subjects (44). In addition, flavonoids may regulate the bacterial flora of the large intestine, leading to altered production of nonflavonoid bacterial products.
Figure 11.
Bacterial metabolites of flavonoids. Isoflavones daidzein (r = H+) and genistein (r = OH−) (A) can undergo reduction to the isoflavonol (B) or the isoflavan (C). The reaction introduces a chiral carbon at position C-3. Metabolism can also lead to the opening of the heterocyclic ring to form desmethylangolensin (D). Further metabolism can result in the formation of the phenolic acids 2-(4-hydroxyphenyl)propionic acid (E) and 4-hydroxyphenylacetic acid (F). Each of these metabolites is colorless.
An unusual class of flavonoids is the C-glycosides. The majority of flavonoid glycosides are O-glycosides (i.e., the bond between the 2 moieties is carbon-oxygen-carbon). In the C-glycosides, the flavonoid and the sugar are joined by a carbon-carbon bond (Fig. 12). Puerarin, the 8-C-glucoside of the isoflavone daidzein, is the principal flavonoid in the subterranean root of the Japanese kudzu vine (Pueraria thunbergiana) (45). Kudzu root is starchy like the potato and is also edible. Unlike the other flavonoids, the C-glycosides of flavonoids may undergo facilitated intestinal and renal tubular transport via GLUT glucose transporters. Puerarin is absorbed rapidly when administered orally and appears in the urine unmetabolized (46). Like most other flavonoids, the C-glycosides are colorless.
Figure 12.
Chemical structures of O- and C-glycosides of flavonoids. Daidzein (O-glycoside) (A) and puerarin (C-glycoside) (B) are found in soy and kudzu root.
Conclusions
Discerning the phytochemicals in fruit and vegetables is limited by our narrow visual appreciation of the world around us. What we see and what we choose to eat are affected by this visual bias. The apparent value of yellow, orange, green, blue, purple, and red vegetables is that we can see them. However, understanding the types of phytochemicals in white vegetables is also important. Horticulturalists currently select white vegetable strains mainly on the basis of their growing characteristics, not their phytochemical content. Processing or cooking white vegetables may have profound effects on their phytochemical content. Therefore, the phytochemical content of vegetables may not provide accurate information on intake. In addition, the extent to which phytochemicals are absorbed after eating and their impact on the gut microbiome cannot be assumed.
Acknowledgments
All authors read and approved the final manuscript.
Literature Cited
- 1.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134(Suppl):3479S–85S [DOI] [PubMed] [Google Scholar]
- 2.Heber D, Bowerman S. Applying science to changing dietary patterns. J Nutr. 2001;131(Suppl):3078S–81S [DOI] [PubMed] [Google Scholar]
- 3.Brand JCD. Lines of light: the sources of dispersive spectroscopy, 1800–1930. 1st ed. Boca Raton (FL): CRC Press; 1995. p. 30–32 [Google Scholar]
- 4.Ogata Y, Kosugi Y. Ultraviolet spectra of L-ascorbic acid and cupric ascorbate complex. Tetrahedron. 1970;26:4711–6 [DOI] [PubMed] [Google Scholar]
- 5.Williams D, Rogers LH. The infrared absorption spectrum of vitamin C. J Am Chem Soc. 1937;59:1422–3 [Google Scholar]
- 6.Stubbs BJ. Captain Cook's beer: the antiscorbutic use of malt and beer in late 18th century sea voyages. Asia Pac J Clin Nutr. 2003;12:129–37 [PubMed] [Google Scholar]
- 7.Campbell JA, Lamar WW. The venomous reptiles of the Western hemisphere. Ithaca (NY): Comstock Publishing Associates; 2004
- 8.Whitehead S, Viner B, Cuddy B, Sullivan K. Dog: the complete guide. London: Team Media Ltd; 1999 [Google Scholar]
- 9.Li X, Li W, Wang H, Bayley DL, Cao J, Reed DR, Bachmanov AA, Huang L, Legrand-Defretin V, Beauchamp GK, et al. Cats lack a sweet taste receptor. J Nutr. 2006;136(Suppl):1932S–4S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quignon P, Kirkness E, Cadieu E, Touleimat N, Guyon R, Renier C, Hitte C, Andre C, Fraser C, Galibert F. Comparison of the canine and human olfactory receptor gene repertoires. Genome Biol. 2003;4:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai T, Sakano H. Odorant receptor-mediated signaling in the mouse. Curr Opin Neurobiol. 2008;18:251–60 [DOI] [PubMed] [Google Scholar]
- 12.Quignon P, Rimbault M, Robin S, Galibert F. Genetics of canine olfaction and receptor diversity. Mamm Genome. 2012;23:132–43 [DOI] [PubMed] [Google Scholar]
- 13.Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt. 1987;26:1437–40 [DOI] [PubMed] [Google Scholar]
- 14.Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193–202 [DOI] [PubMed] [Google Scholar]
- 15.Jacobs GH, Neitz M, Deegan JF, Neitz J. Trichromatic colour vision in New World monkeys. Nature. 1996;382:156–8 [DOI] [PubMed] [Google Scholar]
- 16.Wakakuwa M, Kurasawa M, Giurfa M, Arikawa K. Spectral heterogeneity of honeybee ommatidia. Naturwissenschaften. 2005;92:464–7 [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Carmona M, Sharpe LT, Harlow JA, Barbur JL. Sex-related differences in chromatic sensitivity. Vis Neurosci. 2008;25:433–40 [DOI] [PubMed] [Google Scholar]
- 18.Horton JC. Ocular integration in the human visual cortex. Can J Ophthalmol. 2006;41:584–93 [DOI] [PubMed] [Google Scholar]
- 19.Donaldson MS. A carotenoid health index based on plasma carotenoids and health outcomes. Nutrients. 2011;3:1003–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon YJ, Myung SK, Lee EH, Kim Y, Chang YJ, Ju W, Cho HJ, Seo HG, Huh BY. Effects of beta-carotene supplements on cancer prevention: meta-analysis of randomized controlled trials. Nutr Cancer. 2011;63:1196–207 [DOI] [PubMed] [Google Scholar]
- 21.Hoober JK, Eggink LL, Chen M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth Res. 2007;94:387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–83 [DOI] [PubMed] [Google Scholar]
- 23.Vinson JA, Demkosky CA, Navarre DA, Smyda MA. High-antioxidant potatoes: acute in vivo antioxidant source and hypotensive agent in humans after supplementation to hypertensive subjects. J Agric Food Chem. 2012;60:6749–54 [DOI] [PubMed] [Google Scholar]
- 24.Walter ED. Genistin (an isoflavone glucoside) and its aglucone, genistein, from soybeans. J Am Chem Soc. 1941;63:3273–6 [Google Scholar]
- 25.Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein, and their β-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41:1961–7 [Google Scholar]
- 26.Kim EH, Ro HM, Kim SL, Kim HS, Chung IM. Analysis of isoflavone, phenolic, soyasapogenol, and tocopherol compounds in soybean [Glycine max (L.) Merrill] germplasms of different seed weights and origins. J Agric Food Chem. 2012;60:6045–55 [DOI] [PubMed] [Google Scholar]
- 27.Barnes S, Wang CC, Kirk M, Smith-Johnson M, Coward L, Barnes NC, Vance G, Boersma B. HPLC-mass spectrometry of isoflavonoids in soy and the American groundnut, Apios americana. Adv Exp Med Biol. 2002;505:77–88 [DOI] [PubMed] [Google Scholar]
- 28.Reynolds BD, Blackmon WJ, Wickremesinhe E, Wells MH, Constantin RJ. Domestication of Apios americana. In: Janick J, Simon JE, editors. Advances in new crops. Portland (OR): Timber Press; 1990. p. 436–42.
- 29.Rommens CM, Richael CM, Yan H, Navarre DA, Ye J, Krucker M, Swords K. Engineered native pathways for high kaempferol and caffeoylquinate production in potato. Plant Biotechnol J. 2008;6:870–86 [DOI] [PubMed] [Google Scholar]
- 30.Jung CS, Griffiths HM, De Jong DM, Cheng S, Bodis M, De Jong WS. The potato P locus codes for flavonoid 3′,5′-hydroxylase. Theor Appl Genet. 2005;110:269–75 [DOI] [PubMed] [Google Scholar]
- 31.Andre CM, Oufir M, Guignard C, Hoffmann L, Hausman JF, Evers D, Larondelle Y. Antioxidant profiling of native Andean potato tubers (Solanum tuberosum L.) reveals cultivars with high levels of beta-carotene, alpha-tocopherol, chlorogenic acid, and petanin. J Agric Food Chem. 2007;55:10839–49 [DOI] [PubMed] [Google Scholar]
- 32.Johnston CS, Taylor CA, Hampl JS. More Americans are eating “5 a day” but intakes of dark green and cruciferous vegetables remain low. J Nutr. 2000;130:3063–7 [DOI] [PubMed] [Google Scholar]
- 33.Friedman M. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem. 2006;54:8655–81 [DOI] [PubMed] [Google Scholar]
- 34.Lu S, Van Eck J, Zhou X, Lopez AB, O'Halloran DM, Cosman KM, Conlin BJ, Paolillo DJ, Garvin DF, Vrebalov J, et al. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. Plant Cell. 2006;18:3594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicol Lett. 1995;82–83:173–9 [DOI] [PubMed] [Google Scholar]
- 36.Ho E, Beaver LM, Williams DE, Dashwood RH. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv Nutr. 2011;2:497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradlow HL. Review: indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo. 2008;22:441–5 [PubMed] [Google Scholar]
- 38.Schmid RD, Harborne JB. Mass spectrometric identification of a kaempferol tetraglycoside from Solanum seed. Phytochemistry. 1973;12:2269–73 [Google Scholar]
- 39.Barnes S, Prasain JK, D’Alessandro T, Arabshahi A, Botting N, Lila MA, Jackson G, Janle EM, Weaver CM. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011;2:235–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S [DOI] [PubMed] [Google Scholar]
- 41.Day AJ, Canada FJ, Diaz JC, Kroon PA, Mclauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–70 [DOI] [PubMed] [Google Scholar]
- 42.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127:1260–8 [DOI] [PubMed] [Google Scholar]
- 43.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26 [DOI] [PubMed] [Google Scholar]
- 44.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140(Suppl):1355S–62S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasain JK, Jones K, Kirk M, Wilson L, Smith-Johnson M, Weaver C, Barnes S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2003;51:4213–8 [DOI] [PubMed] [Google Scholar]
- 46.Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004;52:3708–12 [DOI] [PubMed] [Google Scholar]