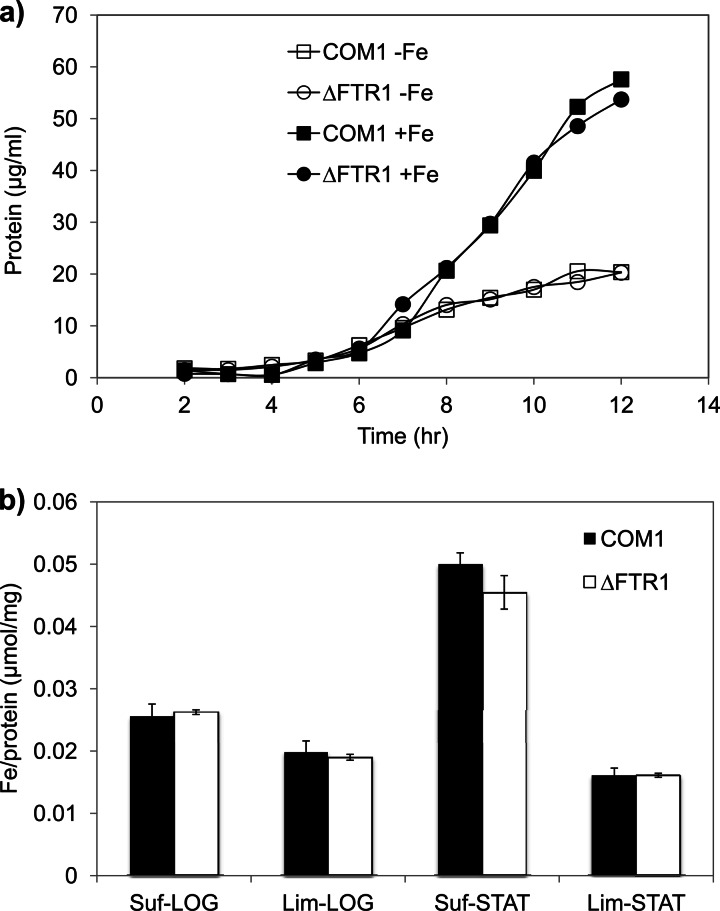
Fig 2.
Characterization of the ΔFTR1 mutant. (a) Growth of ΔFTR1 and COM1 under iron-sufficient [+Fe, 10 μM (NH4)2Fe(SO4)2 added] and iron-limited (−Fe, no iron source added) conditions. Cultures were grown in 125-ml bottles at 98°C with 5 g/liter maltose, 0.5 g/liter yeast extract, and 20 μM uracil. Cell growth was monitored by assaying total cell protein at each time point. Results are shown for COM1 (squares) and ΔFTR1 (circles) under iron-sufficient (closed symbols) and iron-limited (open symbols) conditions. (b) Cultures of ΔFTR1 (open bars) and COM1 (closed bars) grown under iron-limited (Lim) and iron-sufficient (Suf) medium were harvested at exponential (LOG) and stationary (STAT) phases. Cell pellets were washed three times with 1× base salt solution to remove extracellular metals and lysed using lysis buffer. Lysates were then centrifuged, and the supernatant was collected for the measurement of intracellular iron content using ICP-MS. The iron contents measured by ICP-MS were normalized to the protein concentration of each sample.