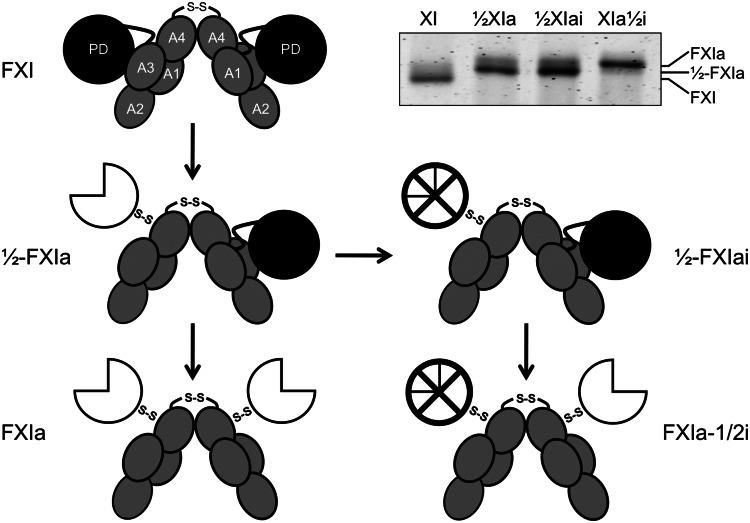
Figure 1.
Schematic diagrams of species generated during fXI activation. The gray ellipses represent the 4 apple domains (A1–A4) and the black circles represent the protease domains (PD) of zymogen fXI. The 2 subunits of the fXI dimer are connected by a hydrophobic interface involving the A4 domains and a disulfide bond involving Cys321. Activation of 1 fXI subunit by cleavage of the Arg369-Ile370 bond results in a species with 1 active site, referred to as 1/2-fXIa. The white three-quarter circle represents the activated protease domain. Subsequent cleavage of the Arg369-Ile370 bond on the second subunit results in generation of fXIa. fXI, 1/2-fXIa, and fXIa migrate slightly differently on SDS-PAGE (inset), facilitating their identification and purification. The preparation of 1/2-fXIa shown in the inset has approximately 10% contamination with fXIa (upper of the 2 bands). Inhibition of the active protease domain (⊗) of 1/2-fXIa yields a species called 1/2-fXIai. Activation of the second subunit of 1/2-fXIai results in a species with 2 activated subunits, 1 of which is inhibited and called fXIa-1/2i.