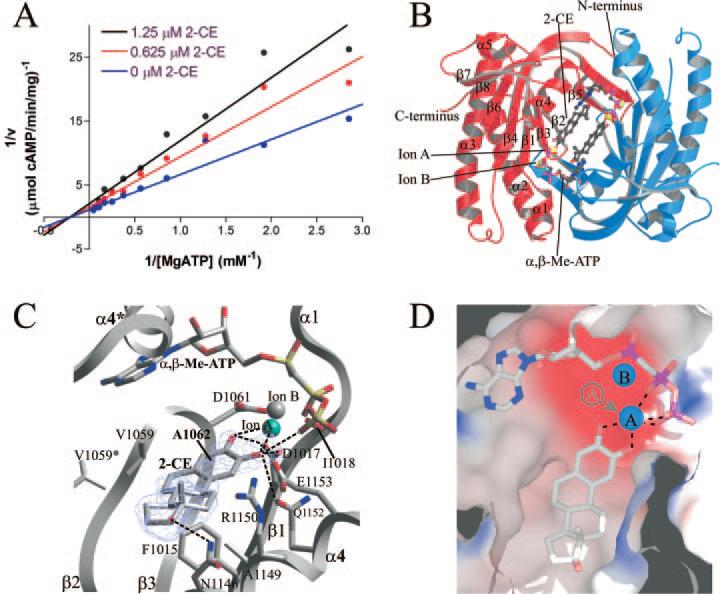
Fig. 3. Structure of CyaC in complex with CE.
A, kinetic mechanism for the inhibition of CyaC by 2-CE. AC activities determined at varying substrate and inhibitor concentrations are displayed in a double reciprocal plot. The linear extrapolations intersect on the x-axis, indicating that CE inhibition is non-competitive with the substrate ATP. B, overall structure of CyaC in complex with the substrate analog α,β-Me-ATP and the inhibitor 2-CE. The two monomers of the homodimer are shown in red and blue, respectively. Two inhibitor molecules occupy the center of the dimer, whereas the active sites are still occupied by the substrate analog and two metal ions. C, detailed view of the interactions between CyaC and the inhibitor 2-CE bound next to the active site, which harbors the substrate analog α,β-Me-ATP and two diva-lent metal ions (one magnesium ion colored cyan, and one calcium ion in gray). Fo – Fc omit electron density for the inhibitor is shown contoured at 2.5 σ. Single letter amino acid abbreviations are presented with position numbers. D, electrostatic surface of the 2-CE binding site showing its mainly hydrophobic nature (blue, positively charged; red, negatively charged). The hydroxyl groups of the inhibitor chelate the catalytic magnesium ion (ion A), removing it from its normal binding site (indicated in gray).