Abstract
Neurologic complications of HIV are well characterized in the central and peripheral nervous systems but not in the autonomic nervous system, perhaps due to the complexities of measuring autonomic function in medically ill populations. We hypothesized that autonomic dysfunction is common in HIV, can be meaningfully measured with an autonomic reflex screen, and is associated with distal symmetric polyneuropathy (DSP) but not with signs of CNS disease. We also sought to characterize immuno-virologic and medical factors associated with autonomic dysfunction. We assessed 102 HIV-infected adults for autonomic dysfunction with a laboratory-based autonomic reflex screen summarized as the Composite Autonomic Severity Score (CASS). The Total Neuropathy Score (TNS) was used to quantify DSP based on neurologic interview/examination, quantitative sensory testing, and nerve conduction studies. Autonomic dysfunction was common, with a CASS ≥ 3 in 61% of participants, of whom 86% were symptomatic. Greater CASS abnormalities demonstrated univariate association with increasing TNS, age, viral load, hypertension, and use of medications (particularly anticholinergics), but not with antiretrovirals, current/nadir CD4+ count, HIV-duration, metabolic factors, or signs of CNS disease. The TNS was the only significant predictor of the CASS in multivariate analysis; anticholinergic medications were marginally significant. This study demonstrates that autonomic dysfunction is common and frequently symptomatic in HIV, and that an autonomic reflex screen, adjusted for anticholinergic medication, is useful in its assessment. Association of autonomic dysfunction with DSP suggests common factors in their pathogenesis, and autonomic neuropathy may be part of the spectrum of HIV-associated peripheral nerve pathologies.
Keywords: autonomic, neuropathy, HIV, anticholinergic
Introduction
Neurologic complications of HIV are well described in the central and peripheral nervous systems and the two most common disorders, HIV-associated neurocognitive disorder and HIV-associated distal symmetric polyneuropathy (DSP), persist despite the use of combination antiretroviral therapy (CART). There is a much poorer understanding of the effects of HIV in the autonomic nervous system, which is likely due to the difficulties inherent in quantifying autonomic signs and symptoms in medically ill populations.
The autonomic nervous system innervates all major organ systems and has a wide array of responsibilities including modulation of heart rate and vasomotor tone, gastrointestinal motility, production of saliva and tears, urination, sexual function, and thermoregulation via sweating. Symptoms of autonomic neuropathy are similarly diverse, and include orthostatic dizziness or fainting, nausea or vomiting especially with meals, diarrhea and/or constipation, dry eyes and mouth, urinary incontinence, sexual dysfunction, and changes in sweating, skin temperature or color (Suarez et al. 1999). In medically ill populations, these symptoms are not easily separated from those of end-organ disease or medication side effects, even with the use of validated questionnaires (Low et al. 2004). Techniques for laboratory testing of autonomic function are well established (Novak 2011); however they also have important limitations in medically ill populations. Non-invasive screening tests rely primarily on the measurement of autonomic reflexes, in particular cardiovascular reflexes and evoked sweat output, both of which may be influenced by concomitant medications (Low and Sletten 2008), and have a limited ability to distinguish central from peripheral autonomic deficits.
Many commonly used medications have potential effects on autonomic testing. In clinical practice, experts recommend discontinuing such medications (anticholinergics, 9-α-fludrocortisone, diuretics, sympathomimetics, parasympathomimetics, and alpha- and beta-blockers) prior to autonomic testing (Low and Sletten 2008). In research, participants taking such medications are often excluded from study. Both approaches are problematic in the context of medically ill individuals with HIV. Withholding medication is potentially deleterious to the patient, and may also cause withdrawal or rebound effects that complicate the interpretation of autonomic test results (Ross et al. 1981). Excluding patients receiving medication results in bias of the study sample toward healthier individuals, which may be unacceptable in the study of chronic illnesses.
Despite these difficulties there has been some preliminary study of autonomic function in HIV using relatively standard, although not identical, batteries of autonomic reflex tests. Studies from early in the AIDS epidemic, prior to the widespread use of CART, suggested that autonomic dysfunction was an important neurologic complication of HIV (Craddock et al. 1987; Freeman et al. 1990; Cohen and Laudenslager 1989; Ruttimann et al. 1991; Villa et al. 1992; Villa et al. 1995). However these studies were small, ranging from five to 57 HIV-infected participants, medication use that could mimic autonomic phenomena was typically not addressed, and in all but one (Freeman et al. 1990), autonomic testing was performed in isolation. Without additional clinical neurologic or neurophysiologic testing, it was unclear if the autonomic dysfunction was part of a larger neuroAIDS syndrome, if it was an isolated nervous system deficit, or if it was potentially a function of confounding medication utilization. Studies from early in the era of CART were similar in results and limitations (Rogstad et al. 1999; Becker et al. 1997; Neild et al. 2000), with the exception of the work of Glück and colleagues who studied 61 HIV-infected participants and documented both autonomic and sensorimotor neuropathy but did not report whether they were correlated.(Gluck et al. 2000) In the last decade, small studies examining predominantly cardiovascular autonomic neuropathy found mostly mild and sub-clinical deficits (Mittal et al. 2004; Lebech et al. 2007; Sakhuja et al. 2007), or symptoms without objective deficits (Compostella et al 2008). More recently Askgaard and colleagues studied 97 HIV-infected participants and found that moderate autonomic dysfunction was associated with medical, but not HIV immuno-virologic variables (Askgaard et al. 2011), a finding replicated by others (Chow et al. 2011).
Autonomic dysfunction may have particular relevance to CART-treated HIV, as it has been hypothesized that lipodystrophy may be the result of a selective autonomic neuropathy (Fliers et al. 2003a) based on the role of the autonomic nervous system in determination of body composition (Fliers et al. 2003b) and experimental data demonstrating lower parasympathetic cardiac modulation in HIV patients with lipodystrophy (Chow et al. 2006). Other authors have proposed that the autonomic nervous system might directly affect immune function in HIV via sympathetic innervation of lymph nodes (Sloan et al. 2007). In the rhesus macaque model, there is a complicated interaction between simian immunodeficiency virus (SIV) and autonomic innervation, with an overall decrease in sympathetic innervation of lymph nodes following SIV infection, but enhanced viral replication adjacent to the remaining sympathetic varicosities (Sloan et al. 2008; Sloan et al. 2006). Differential immune reconstitution in response to ART has also been reported in humans based on autonomic function (Cole et al. 2001).
Thus, the clinical relevance of autonomic dysfunction to HIV argues for the need to determine whether it is common and whether it is part of a larger neurologic syndrome. Considering the known high prevalence of HIV-DSP, and the association of autonomic neuropathy with DSP in other systemic diseases such as diabetes, we hypothesized that autonomic dysfunction would be common in HIV and associated with DSP but not clinical signs of CNS disease. We further hypothesized that the following factors might be important covariates in this association: CART, HIV duration, current and nadir CD4 count, HIV plasma load, and based on the findings of Askgaard and colleagues, features of the metabolic syndrome. We also anticipated that our patients would be on multiple medications and sought to determine the effect of this polypharmacy on the results of autonomic testing. Finally we sought to describe the autonomic symptoms experienced by HIV-infected patients.
Methods
Recruitment
One hundred and two HIV-infected individuals were recruited from the waiting room of an adult HIV clinic, in an academic medical center, in which all clinical information is housed in an electronic medical record (EMR). Approximately 1500 low-income, predominantly minority patients are followed in the clinic. All recruitment occurred between April 2011 and August 2012. Most visits to the clinic are for primary HIV care, although ancillary services (nursing, social work, and care coordination) and limited sub-specialty care (psychiatry, psychology, nephrology, gynecology, and neurology) are also offered on site. In order to avoid overrepresentation of patients with neurologic disorders, recruitment was not performed when a neurologist was seeing patients. The investigator recruiting participants had no specific knowledge of their medical conditions, and offered an appointment to assess eligibility to any willing patient. All procedures were performed according to a protocol approved by the Mount Sinai School of Medicine Institutional Review Board. All participants provided written informed consent.
Inclusion/exclusion criteria
All HIV-infected individuals, 18 years of age or greater, receiving care in the clinic were eligible to participate. The following medical conditions were exclusionary because they might preclude safe and/or accurate autonomic testing: glaucoma, aortic stenosis, myocardial infarction within 6 months, retinopathy (Hebert et al. 1998; Airaksinen et al. 1993), unclipped cerebral aneurysm (Tiecks et al. 1996) cardiac arrhythmias, or pacemakers. Participants with other potential causes of autonomic dysfunction were not excluded because we wanted our sample to be as representative of the clinic population as possible.
Testing procedures
All participants were seen for a single visit between May 2011 and August 2012. Each of the study procedures was performed by the same investigator for all participants. The EMR was reviewed to confirm co-morbid medical conditions, current medications, current and nadir CD4+ count, viral load, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides and random glucose (fasting glucose was not routinely available for all patients). Laboratory values had been collected within one month of the study visit for 78% of participants, and within 4 months for 98%. A standardized comprehensive neurologic examination; the Survey of Autonomic Symptoms (SAS) (Zilliox et al. 2011, 1099–1105); quantitative sensory testing (QST) of cooling and vibration in the right foot (CASE IV system); and nerve conduction studies (right peroneal motor and sural sensory) were performed.
Autonomic function was assessed using the autonomic reflex screen described by Low and colleagues (Novak 2011). This screening battery was chosen because it is non-invasive and can be used to calculate a validated quantitative measure of autonomic dysfunction, the Composite Autonomic Severity Score (CASS) (Low 1993). Autonomic testing was performed using equipment from WR Medical Electronics Co. (Maplewood, MN). The autonomic reflex screen includes four tests: quantitative measurement of sweat output at four standardized sites (foot, distal leg, proximal leg and forearm) evoked by iontophoresis of acetylcholine into the skin (Q-sweat), heart rate variability in response to paced rhythmic deep breathing, heart rate and blood pressure response to Valsalva maneuver, and heart rate and blood pressure response to tilt table testing (Low 1993). Participants were advised to refrain from smoking and caffeine consumption on the morning of testing.
Calculation of measures
The total neuropathy score (TNS) was used as the measure of DSP. The TNS is calculated from the following 10 items scored from 0–4, where 0 is normal: sensory symptoms, motor symptoms, autonomic symptoms, pin sensibility, vibration sensibility, strength, deep tendon reflexes, QST vibration, sural sensory amplitude and peroneal motor amplitude (Cornblath et al. 1999). Two minor modifications were necessary: the QST scoring was modified for the CASE IV system (Simpson et al. 2006) and the SAS was used to quantify autonomic symptoms (Zilliox et al. 2011). Recognizing that including autonomic symptoms in the TNS might artificially enhance its association with the CASS, we repeated all relevant analyses with a modified TNS that excluded autonomic symptoms. The CASS and its three sub-scores (sudomotor, adrenergic, and cardiovagal) were calculated using the data from the autonomic testing (Low 1993). The sudomotor sub-score reflects sympathetic innervation of sweat glands and has a possible range of 0–3, the cardiovagal sub-score reflects parasympathetic innervation of the heart and has a possible range of 0–3, the adrenergic sub-score reflects sympathetic innervation of peripheral vasculature and has a possible range of 0–4. The total CASS is the sum of the three sub-scores, resulting in a total score of 0–10, where zero is normal. The following definitions have been used for CASS scores greater than zero: 0–1 normal; 2–3 mild autonomic dysfunction; 4–6 moderate autonomic dysfunction; 7–10 severe autonomic dysfunction (Low et al. 2004). We chose a relatively stringent cut-off of ≥3 to define autonomic dysfunction in acknowledgement of the medical complexity of our population.
The HIV-Dementia Motor Score (HDMS) was calculated as a clinical measure of HIV-related CNS dysfunction (Robinson-Papp et al. 2008). The HDMS is a validated measure, designed to quantify the clinical neurologic findings of HIV-associated dementia (Byrd et al. 2013). It is scored on a scale of 0–20, where zero is normal, and includes: limb weakness, hyperreflexia, abnormal reflexes (snout, glabellar and Babinski sign), hypertonia, dysmetria and gait abnormality.
Assessing medication affects
The following classes of medications have the potential to interfere with the results of autonomic testing: anticholinergics, diuretics, 9-α-fludrocortisone, sympathomimetics, parasympathomimetics, alpha blockers and beta blockers. Since acetylcholine is the preganglionic neurotransmitter for both parasympathetic and sympathetic neurons and the postganglionic neurotransmitter for parasympathetic and sympathetic sudomotor neurons, anticholinergics have the potential for widespread effects on autonomic testing, and in particular the sudomotor sub-score of the CASS. The other agents might be expected to affect the cardiovagal and adrenergic sub-scores of the CASS. We used the Anticholinergic Risk Scale (ARS) to quantify the burden of anticholinergic medications for each patient (Rudolph et al. 2008). This scale provides a validated, quantitative measure of the cumulative anticholinergic effects of commonly used medications and is suitable for use in the setting of polypharmacy. Unfortunately such scales do not exist for the other classes of medications listed above, and so we considered them individually.
Statistical Analysis
The pre-planned primary outcome was the Spearman's rank correlation between the CASS and TNS. Other outcomes were secondary and a correction for multiple comparisons (e.g. the Bonferroni correction factor) was not applied, thus these analyses must be considered exploratory. Kendall's tau-b correlation was performed for the TNS and each CASS sub-score. Spearman's rank correlation was performed for the CASS and each of the following: age, duration of HIV infection, CD4+ count (current and nadir), serum HIV viral load, random glucose, total cholesterol, LDL, HDL, triglycerides, and body mass index (BMI). In addition, the median CASS was compared between genders (Wilcoxon rank sum test) and across ethnicities (Kruskal-Wallis test). The Wilcoxon rank sum test was also used to compare the median CASS in participants with and without: current CART treatment; obesity; diagnoses of diabetes, hypertension, or hyperlipidemia. In order to determine the effect of medication on CASS, Spearman's rank correlation was calculated between the ARS and the CASS, and Kendall's tau-b correlation was performed for the ARS and each CASS sub-score. The Wilcoxon rank sum test was also used to compare the median CASS between patients who were and were not taking the other medication classes of interest. Chi-square analysis was used to compare the proportion of participants with cardiovagal and adrenergic sub-score abnormalities based on whether or not they were receiving the other medication classes of interest. Linear regression was used to determine whether any of the above factors were important confounders in the relationship between the TNS and CASS. All tests were two-tailed and conducted at the α = 0.05 level using SPSS version 20.
Results
Participant characteristics
Participants were predominantly minority, with a nearly equal gender distribution (table 1). Most had longstanding HIV and were prescribed CART. Metabolic co-morbidities were fairly common, with hypertension the most common (42%). Thirteen participants had a history of diabetes, and 53 had a history of substance use, but there were no other diagnoses recorded in the EMR that would account for autonomic dysfunction or DSP (e.g. Sjogren's syndrome, autoimmune autonomic ganglionopathy, pure autonomic failure, or inherited neuropathies). Among the 13 diabetics, 12 had active type 2 diabetes and one had a history of gestational diabetes. Most were relatively recently diagnosed, with a median duration of 5 years (IQR 3, 8). None had end-organ complications of diabetes. Among those with a substance use history, 13 had stimulant (cocaine, amphetamines or phencyclidine) use documented in their clinic chart within two months of the study visit (either by urine toxicology or health care provider note).
Table 1.
Participant characteristicsa
| N | 102 |
|---|---|
|
| |
| Gender | |
| Male | 52% |
| Female | 48% |
|
| |
| Ethnicity | |
| African-American | 62% |
| Hispanic | 32% |
| White | 6% |
|
| |
| Age (years) | 51 (44, 57) |
|
| |
| Prescribed antiretrovirals | 95% |
|
| |
| Duration of known HIV infection (years) | 16 (13, 22) |
|
| |
| CD4+ count (cells/mm3) | 439 (288, 702) |
|
| |
| Detectable HIV viral load (>50 copies/ml) | 42% |
|
| |
| Co-morbid metabolic conditions | |
| Diabetes | 13% |
| Hypertension | 42% |
| Hyperlipidemia | 16% |
| Obesity | 15% |
Values are median (IQR) unless expressed as a percentage.
The majority of participants were taking traditional CART regimens with nucleoside reverse transcriptase inhibitor (NRTI) backbones and protease inhibitor (PI, 52%), non-nucleoside reverse transcriptase inhibitor (NNRTI, 18%) or integrase inhibitor (13%) bases. Other regimens were each taken by less than 5% of participants and included: PI/integrase inhibitor, NRTI/PI/integrase inhibitor, NRTI only, NRTI/PI/entry inhibitor, NRTI/integrase inhibitor/entry inhibitor. Five percent of participants were not taking any CART. Although 42% of participants had detectable viral loads, this was mostly low level viremia; 89% of treated patients had <5000 copies.
Description of measures
Abnormalities on the CASS were very common (table 2), with 62 (61%) participants meeting criteria for autonomic dysfunction (CASS ≥ 3). Most deficits were mild to moderate, with 94% of participants receiving a score of 1–5 out of 10. Deficits were seen in all three autonomic sub-scores (sudomotor, adrenergic, cardiovagal), but were most prominent in the sudomotor sub-score. Eighty-two percent of participants had sudomotor deficits, and all levels of severity were represented (scores of 1–3). Adrenergic deficits were seen in 75% of participants, but nearly all of these were at the lowest severity level (1 out of 4). Cardiovagal deficits were seen in 37% of participants, again nearly all at the lowest severity level (1 out of 3). The most common cardiovascular reflex abnormalities were: excessive blood pressure drop during Valsalva maneuver (63%), blunted compensatory tachycardia in response to blood pressure drop during Valsalva maneuver (i.e. reduced Valsalva ratio; 28%), and reduced heart rate variability in response to paced rhythmic deep breathing (26%). Distal symmetric polyneuropathy was also very common. Very few participants (4%) had a completely normal TNS. Fifty percent of participants had at least three of the following signs of neuropathy: sensory symptoms, sensory signs, reduced or absent ankle reflexes, and reduced or absent sural sensory amplitude. Twenty-nine percent had all four. The TNS was not associated with obesity, diabetes or hyperlipidemia (p>.2 for all variables). The TNS was associated with hypertension suggesting a possible role for co-morbid illness in the etiology of DSP in addition to HIV itself (W (n1 = 43, n2 = 59) = 2729, p=.04).
Table 2.
Distribution of measures
| Median (IQR) | Observed range | Possible range | |
|---|---|---|---|
| CASS total | 3 (2, 4) | 0–6 | 0–10 |
| TNS | 8 (4, 13) | 0–23 | 0–40 |
| SAS symptom number | 2 (1, 4) | 0–9 | 0–12 |
| SAS impact score | 8 (1, 15) | 0–45 | 0–60 |
Abbreviations: composite autonomic severity score (CASS), total neuropathy score (TNS), survey of autonomic symptoms (SAS)
Main outcomes
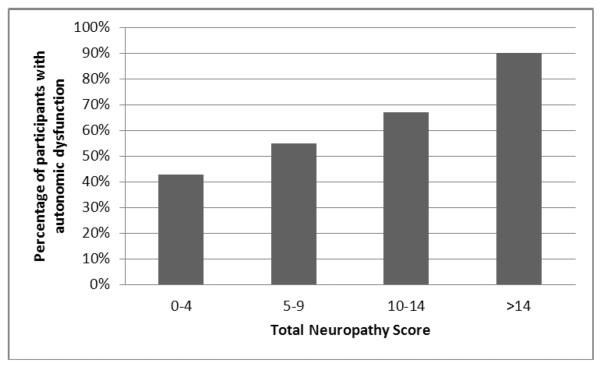
The CASS and TNS were correlated in the expected direction (r=.33, p=.001). This result was unchanged when autonomic symptoms were excluded from the TNS (r=.33, p=.001). A large majority (90%) of participants with the most severe DSP (TNS ≥ 15) had autonomic dysfunction (CASS ≥ 3), whereas only 30% of participants with little or no neuropathy (TNS < 4) had autonomic dysfunction (see figure).
Figure.
The percentage of participants with autonomic dysfunction, defined as a Composite Autonomic Severity Score ≥ 3, increases with distal symmetric polyneuropathy severity as measured by the Total Neuropathy Score.
The CASS sudomotor and adrenergic sub-scores were also correlated with the TNS (r=.19, p=.01; r=.18, p=.03 respectively) and the cardiovagal sub-score was marginally correlated with the TNS (r=.16, p=.06). Greater abnormalities on the CASS were also correlated with increasing age (r=.26, p=.009), increasing viral load (r=.20, p=.04), and a diagnosis of hypertension (W (n1 = 43, n2 = 59) = 2814, p=.004). The CASS was not associated with clinical signs of CNS dysfunction (as measured by the HDMS), gender, ethnicity, HIV duration, diabetes, obesity, hyperlipidemia, BMI, or the following laboratory values: current or nadir CD4, total cholesterol, LDL, HDL, triglycerides and random glucose (all p values >.1).
Effects of medication use on the CASS
The CASS was not associated with CART, either overall or by regimen type. Thirty-seven participants were using one or more medications with anticholinergic properties, 14 were using diuretics, and 12 were using beta-blockers. Clonidine and an alpha-blocker (terazosin) were each used by one participant. Two participants were prescribed sympathomimetics (pseudoephedrine and modafinil) and 13 participants had evidence of recent illicit sympathomimetic use (cocaine, amphetamines or phencyclidine). There were no participants taking 9-α-fludrocortisone, direct parasympathomimetics, or acetylcholinesterse inhibitors. Two participants used nicotine patches, an indirect parasympathomimetic. The ARS was correlated with the CASS (r=.26, p=.01). This association was driven primarily by the sudomotor sub-score (r=.23, p=.007). The ARS was not correlated with the adrenergic or cardiovagal sub-scores (p>.1 for both). Due to the infrequency with which the other agents were used, only sympathomimetics (prescribed and illicit), diuretics and beta-blockers were considered further. Sympathomimetic use was not associated with the CASS (p>.8). The use of a diuretic was associated with a higher median CASS (4 vs. 3; W (n1 = 88, n2 = 14) = 4250, p=.005). Participants using a beta-blocker also had a slightly higher median CASS (3.5 vs. 3) but this was not statistically significant (p=.2). Both diuretics (χ2=5.1, p=.02) and beta-blockers (χ2=8.3, p=.004) were associated with abnormalities on the cardiovagal sub-score, but neither was associated with abnormalities on the adrenergic subscore (p>.1 for both). Since diuretics and beta-blockers had similar effects on the autonomic testing they were considered together in the multivariate analysis.
Multivariate analysis
Multivariate linear regression was performed to determine whether the TNS was associated with the CASS after adjustment for the following potential confounders: age, viral load, hypertension, the ARS, beta-blocker or diuretic use. The TNS was the only statistically significant predictor in the model (β=.07, standardized β=.26, p=.01), although the ARS was marginally significant (β=.19, standardized β=.16, p=.09). The results were not changed if autonomic symptoms were excluded from the TNS.
Analyses of autonomic symptoms
Eighty-six percent of participants with autonomic dysfunction (defined as CASS ≥ 3) endorsed one or more symptoms on the SAS and 70% had at least one symptom they described moderately bothersome or worse. The most common symptoms were lightheadedness, dry eyes or mouth, cold feet, and constipation, each occurring in at least 40% of participants (see table 3). The presence of one or more symptoms that were at least moderately bothersome on the SAS was associated with autonomic dysfunction (χ2(1)=6.0, p=.014). Overall the CASS was marginally associated with both the SAS impact score and the SAS symptom number (r=.18, p=.07; r=.17, p=.08 respectively). However only two individual symptoms (cold feet and constipation) were associated with autonomic dysfunction, which reflects the non-specific nature of individual autonomic symptoms in isolation and the high symptom burden of HIV-infected populations even without autonomic dysfunction.
Table 3.
Individual autonomic symptoms in participants with objective signs of autonomic dysfunction (Composite Autonomic Severity Score ≥ 3)
| Autonomic Symptom | Percentage who experience the symptom |
|---|---|
| Lightheadedness | 48% |
| Dry mouth or dry eyes | 45% |
| Feet colder than rest of body | 48% |
| Constipation | 42% |
| Erectile dysfunction (men only) | 29% |
| Nausea, vomiting or bloating after a small meal | 24% |
| Urinary incontinence | 24% |
| Diarrhea | 10% |
| Decreased sweating in feet compared to rest of body | 16% |
| Decreased sweating in feet following exercise or in hot weather | 10% |
| Increased sweating in hands | 7% |
| Pale or blue feet | 10% |
Discussion
A growing body of evidence suggests that mild to moderate autonomic dysfunction frequently accompanies HIV infection; how this fits in to the spectrum of CART-era neuroAIDS disorders is unclear. Prior studies have employed autonomic reflex tests including measures of heart rate variability, pupillary constriction, heart rate and blood pressure responses to the Valsalva maneuver, sustained handgrip, and standing; but few have included any assessment of neurologic deficits outside of the autonomic nervous system. Furthermore, many studies have not addressed the effect of confounding medications on reflex testing. Two recent CART-era studies attempted to describe autonomic dysfunction in a neurologic context, but neither performed a comprehensive autonomic or medication utilization assessment. In one of these studies, we reviewed the records of 168 HIV-infected participants in a longitudinal neuroAIDS (Manhattan HIV Brain Bank) cohort and found that autonomic-type symptoms were associated with clinically diagnosed DSP (Robinson-Papp et al. 2012). In the other study, Boger and colleagues performed quantitative sudomotor axon reflex testing in 41 HIV-infected individuals and found that sweat volumes were lower in participants with DSP on clinical examination (Boger et al. 2012).
The current study confirms the high frequency of HIV-associated autonomic dysfunction, and in addition finds that the strongest and most consistent predictor of autonomic dysfunction in HIV is peripheral nerve dysfunction. Autonomic dysfunction was present in up to 90% of participants with severe DSP and in only 30% of participants with little or no DSP. This finding demonstrates that autonomic dysfunction should be considered a common part of the HIV-DSP syndrome and suggests that autonomic dysfunction and DSP may have common underlying pathogenetic mechanisms in HIV. It is as yet unclear what these mechanisms are. The pathogenesis of DSP in HIV is incompletely understood. Proposed mechanisms include mitochondrial dysfunction and energetic failure in the distal axon (Lehmann et al. 2011) and gp-120 induced neurotoxicity (Keswani et al. 2003; Melli et al. 2006). The pathogenesis of autonomic dysfunction in HIV is even less well understood. There is sympathetic denervation of lymph nodes in the rhesus macaque following acute SIV infection (Sloan et al. 2008), but lymph nodes contain a high concentration of virally infected cells and it is unknown if autonomic innervation of other organs is similarly affected. Autonomic nerve fibers are predominantly small caliber, similar to somatic fibers that are often affected in DSP. Thus it seems reasonable that both groups of fibers would be vulnerable to the same insults, but this has yet to be proven.
Our study also shows that although medications, particularly those with anticholinergic properties, affect the outcome of autonomic function tests, this affect is not large and can be accounted for using the ARS. According to the multivariate analysis described above, the slope of the correlation between the ARS and the CASS was .19, and so in general, approximately five points on the ARS would be expected to raise the CASS by one point, most likely in the sudomotor sub-score. An ARS of five would be equivalent to one medication with very strong anticholinergic properties and a second one with strong anticholinergic properties (e.g. diphenhydramine and nortiptyline). We did not find an association between the CASS and sympathomimetic use. This may be due to the imprecision inherent in relying on chart review for this information. Future studies should include urine toxicology, and other important laboratory measures (e.g. CD4 count, viral load, metabolic indices) contemporaneous with the autonomic testing.
Autonomic dysfunction was not only common in this population of HIV-infected individuals but also highly symptomatic. A population based study of diabetics that used the same diagnostic methods found a mean CASS of 3.4, and a CASS of ≥ 2 in 54% of type 1 diabetics and 73% of type 2 diabetics.(Low et al. 2004; Suarez et al. 2005) Seventy-eight percent of our participants met the same criteria. Using a slightly more stringent criteria of a CASS ≥ 3, 61% of our participants had autonomic dysfunction, of whom 86% were symptomatic. Interestingly neither autonomic dysfunction nor DSP was associated with diabetes in our study, likely because the diabetes was mild.
This study has potentially important implications. DSP is a significant source of pain and diminished quality of life for many HIV-infected individuals (Ellis et al. 2010). However due to its predominantly sensory and non-progressive nature (Simpson et al. 2006), it has not generally been considered disabling or relevant to the course of systemic disease. The finding that HIV-DSP is associated with autonomic dysfunction changes this perception. In diabetes, cardiovascular autonomic neuropathy is associated with increased mortality (Maser et al. 2003; Pop-Busui et al. 2010) It is yet unknown if this is also the case in HIV.
The potential clinical implications of autonomic neuropathy become increasingly important in light of the aging of the HIV-infected population. We found that increasing age was associated with worsening autonomic deficits, but was not an independent risk factor. This is likely due to the fact that age is also strongly associated with HIV-DSP. Nonetheless worsening autonomic function can be expected with increasing age. The median age in our sample was 51 years, with 75% of participants 57 years or younger and autonomic dysfunction was very common even in this relatively young group. Aging and increasing severity of autonomic dysfunction may have multiple harmful synergies. Cardiovascular disease increases with age, as do changes in body composition, known as sarcopenic obesity of aging (Boirie 2009). Poorer immunologic response to CART has also been observed in older patients (Sabin et al. 2008), potentially due to immunosenescence (Deeks 2011).
This study has limitations. Although we reviewed the EMR for other diagnoses associated with autonomic neuropathy, we did not perform specific testing (e.g. measurement of vitamin levels) to exclude other causes. All participants were recruited from a single clinic in an academic medical center known to have expertise in the field of neuroAIDS. Accordingly our participants may be more medically and neurologically ill than most HIV patients. These factors may limit generalizability. Due to financial constraints, blood test results were taken from clinic records and so are not exactly contemporaneous with the study visit. Since our hypothesis was that autonomic dysfunction would be associated with DSP, we did not have a comprehensive CNS evaluation (e.g. neuropsychological testing or neuroimaging). Finally, as our primary objective was to screen for a spectrum of autonomic abnormalities in a medically ill population, we did not employ invasive or interventional tests. While such tests have the advantage of potentially greater specificity, they each have their own limitations. For example, direct measurement of autonomic activity from peripheral nerves is possible using needle electrodes (microneurography) (Vallbo et al 2004). However the technique measures only sympathetic and not parasympathetic activity and responses may be difficult to obtain in patients with neuropathy. Imaging modalities such as MIBG-SPECT have been used to assess adrenergic sympathetic terminals in the heart (Dae et al. 1989), but this does not provide information about parasympathetic function or innervation of other structures. Pharmacologically based methods measure changes in blood pressure and heart rate during intravenous infusion of various agents (e.g. phenylephrine, tyramine, isoproterenol) (La Rovere et al. 1988; Almquist et al. 1989; Demanet 1976) or compare levels of endogenous serum catecholamines during orthostatic or pharmacologic challenge (Polinsky et al. 1981). Unlike the reflex based tests, pharmacologic methods may provide more insight as to whether an autonomic deficit is central or peripheral, but they do not eliminate the confounding effects of polypharmacy and likely carry too much risk for research in vulnerable populations.
An important strength of this study is that, to our knowledge, it is the first to demonstrate an association of DSP with autonomic dysfunction in HIV, and is one of the largest studies of autonomic function in HIV to date. Another strength is rigorous standardization of methods and quality control. All assessments were done by one of two physicians (JRP or SS), with all neurologic examinations and nerve conductions done by an attending neurologist board certified in electrodiagnostic medicine (JRP), who was blind to the results of the autonomic testing while performing these assessments. All data was reviewed for accuracy by two authors at the time of the study visit and then again at a later date, resulting in a total of at least four data reviews.
In summary, our study demonstrates that a non-invasive, laboratory-based autonomic reflex screen is useful in the detection of autonomic neuropathy in HIV and that although medications, particularly anticholinergics, do affect the testing, this affect can be accounted for using the Anticholinergic Risk Scale. Using this autonomic reflex screen, we found that autonomic dysfunction is common in HIV, often symptomatic, and is associated with DSP but not with clinical signs of CNS dysfunction. Furthermore greater autonomic dysfunction is observed in older participants and in those with higher viral loads. Further research is indicated to explore the pathophysiology underlying HIV associated DSP and autonomic dysfunction, and their clinical implications.
Acknowledgements
This publication was supported by a grant (K23 NS066789) from the National Institute of Neurological Disorders and Stroke (NINDS) to Dr. Robinson-Papp. The authors thank biostatisticians James Godbold PhD and Emilia Bagiella PhD of the Mount Sinai School of Medicine for statistical assistance.
References
- Airaksinen KE, Hartikainen JE, Niemela MJ, Huikuri HV, Mussalo HM, Tahvanainen KU. Valsalva manoeuvre in the assessment of baroreflex sensitivity in patients with coronary artery disease. Eur Heart J. 1993;14:1519–23. doi: 10.1093/eurheartj/14.11.1519. [DOI] [PubMed] [Google Scholar]
- Almquist A, Goldenberg IF, Milstein S, Chen MY, Chen XC, Hansen R, Gornick CC, Benditt DG. Provocation of bradycardia and hypotension by isoproterenol and upright posture in patients with unexplained syncope. New Engl J Med. 1989;320:346–51. doi: 10.1056/NEJM198902093200603. doi: 10.1056/NEJM198902093200603. [DOI] [PubMed] [Google Scholar]
- Askgaard G, Kristoffersen US, Mehlsen J, Kronborg G, Kjaer A, Lebech AM. Decreased heart rate variability in HIV positive patients receiving antiretroviral therapy: Importance of blood glucose and cholesterol. PloS One. 2011;6:e20196. doi: 10.1371/journal.pone.0020196. doi: 10.1371/journal.pone.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Gorlach I, Frieling T, Haussinger D. Characterization and natural course of cardiac autonomic nervous dysfunction in HIV-infected patients. AIDS. 1997;11:751–7. doi: 10.1097/00002030-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Boger MS, Hulgan T, Haas DW, Mitchell V, Smith AG, Singleton JR, Peltier AC. Measures of small-fiber neuropathy in HIV infection. Auton Neurosci. 2012;169:56–61. doi: 10.1016/j.autneu.2012.04.001. doi: 10.1016/j.autneu.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr. 2009;13:717–23. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- Byrd DA, Robinson-Papp J, Rivera Mindt M, Mintz L, Elliott K, Lighty Q, Morgello S, the Manhattan HIV Brain Bank Isolating cognitive and neurologic HIV effects in substance-dependent, confounded cohorts: A pilot study. JINS. 2013 doi: 10.1017/S1355617712001634. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DC, Wood R, Choi J, Grandinetti A, Gerschenson M, Sriratanaviriyakul N, Nakamoto B, Shikuma C, Low P. Cardiovagal autonomic function in HIV-infected patients with unsuppressed HIV viremia. HIV Clin Trials. 2011;12:141–50. doi: 10.1310/hct1203-141. doi: 10.1310/hct1203-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DC, Wood R, Grandinetti A, Shikuma C, Schatz I, Low P. Cardiovagal autonomic dysfunction in relation to HIV-associated lipodystrophy. HIV Clin Trials. 2006:16–23. doi: 10.1310/B6PT-56HG-K3DN-84KK. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Laudenslager M. Autonomic nervous system involvement in patients with human immunodeficiency virus infection. Neurology. 1989;39:1111–2. doi: 10.1212/wnl.39.8.1111. [DOI] [PubMed] [Google Scholar]
- Cole SW, Naliboff BD, Kemeny ME, Griswold MP, Fahey JL, Zack JA. Impaired response to HAART in HIV-infected individuals with high autonomic nervous system activity. PNAS. 2001;98:12695–700. doi: 10.1073/pnas.221134198. doi: 10.1073/pnas.221134198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compostella C, Compostella L, D'Elia R. The symptoms of autonomic dysfunction in HIV-positive Africans. Clin Auton Res. 2008;18:6–12. doi: 10.1007/s10286-007-0451-y. doi: 10.1007/s10286-007-0451-y. [DOI] [PubMed] [Google Scholar]
- Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T. Total neuropathy score: Validation and reliability study. Neurology. 1999;53:1660–4. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- Craddock CG, Pasvol R, Bull A, Protheroe A, Hopkin J. Cardiorespiratory arrest and autonomic neuropathy in AIDS. Lancet. 1987;2:16–8. doi: 10.1016/s0140-6736(87)93054-6. [DOI] [PubMed] [Google Scholar]
- Dae MW, O'Connell JW, Botvinick EH, Ahearn T, Yee E, Huberty JP, Mori H, Chin MC, Hattner RS, Herre JM. Scintigraphic assessment of regional cardiac adrenergic innervation. Circulation. 1989;79:634–44. doi: 10.1161/01.cir.79.3.634. [DOI] [PubMed] [Google Scholar]
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. doi:10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demanet JC. Usefulness of noradrenaline and tyramine infusion tests in the diagnosis of orthostatic hypotension. Cardiology. 1976;61:213–24. doi: 10.1159/000169812. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: The CHARTER study. Arch Neurol. 2010;67:552–8. doi: 10.1001/archneurol.2010.76. doi:10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Kreier F, Voshol PJ, Havekes LM, Sauerwein HP, Kalsbeek A, Buijs RM, Romijn JA. White adipose tissue: Getting nervous. J Neuroendocrinol. 2003;15:1005–10. doi: 10.1046/j.1365-2826.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- Fliers E, Sauerwein HP, Romijn JA, Reiss J, van der Valk M, Kalsbeek A, Kreier F, Buijs RM. HIV-associated adipose redistribution syndrome as a selective autonomic neuropathy. Lancet. 2003;362:1758–60. doi: 10.1016/s0140-6736(03)14858-1. [DOI] [PubMed] [Google Scholar]
- Freeman RM, Roberts S, Friedman LS, Broadbridge C. Autonomic function and human immunodeficiency virus infection. Neurology. 1990;40:575–80. doi: 10.1212/wnl.40.4.575. [DOI] [PubMed] [Google Scholar]
- Gluck T, Degenhardt E, Scholmerich J, Lang B, Grossmann J, Straub RH. Autonomic neuropathy in patients with HIV: Course, impact of disease stage, and medication. Clin Auton Res. 2000;10:17–22. doi: 10.1007/BF02291385. [DOI] [PubMed] [Google Scholar]
- Hebert JL, Coirault C, Zamani K, Fontaine G, Lecarpentier Y, Chemla D. Pulse pressure response to the strain of the Valsalva maneuver in humans with preserved systolic function. J Appl Physiol. 1998;85:817–23. doi: 10.1152/jappl.1998.85.3.817. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–96. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Specchia G, Mortara A, Schwartz JP. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation. 1988;78:816–24. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- Lebech AM, Kristoffersen US, Mehlsen J, Wiinberg N, Petersen CL, Hesse B, Gerstoft J, Kjaer A. Autonomic dysfunction in HIV patients on antiretroviral therapy: Studies of heart rate variability. Clin Physiol Funct Imaging. 2007;27:363–7. doi: 10.1111/j.1475-097X.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- Lehmann HC, Chen W, Borzan J, Mankowski JL, Hoke A. Mitochondrial dysfunction in distal axons contributes to human immunodeficiency virus sensory neuropathy. Ann Neurol. 2011;69:100–10. doi: 10.1002/ana.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;8:748–52. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- Low PA, Sletten DM. Laboratory evaluation of autonomic failure. In: Low PA, Benarroch EE, editors. Clinical autonomic disorders. 3rd edn Lippincott Williams & Wilkins; Baltimore: 2008. pp. 130–160. [Google Scholar]
- Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: A population-based study. Diabetes Care. 2004;27:2942–7. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: A meta-analysis. Diabetes Care. 2003;26:1895–901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- Melli G, Keswani SC, Fischer A, Chen W, Hoke A. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129:1330–8. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- Mittal CM, Wig N, Mishra S, Deepak KK. Heart rate variability in human immunodeficiency virus-positive individuals. Int J Cardiol. 2004;94:1–6. doi: 10.1016/j.ijcard.2003.02.002. doi: 10.1016/j.ijcard.2003.02.002. [DOI] [PubMed] [Google Scholar]
- Neild PJ, Amadi A, Ponikowski P, Coats AJ, Gazzard BG. Cardiac autonomic dysfunction in AIDS is not secondary to heart failure. Int J Cardiol. 2000;74:133–7. doi: 10.1016/s0167-5273(00)00232-1. [DOI] [PubMed] [Google Scholar]
- Novak P. Quantitative autonomic testing. JoVE. 2011;53:2502. doi: 10.3791/2502. doi: 10.3791/2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polinsky RJ, Kopin IJ, Ebert MH, Weise V. Pharmacologic distinction of different orthostatic hypotension syndromes. Neurology. 1981;31:1–7. doi: 10.1212/wnl.31.1.1. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R. Effects of cardiac autonomic dysfunction on mortality risk in the action to control cardiovascular risk in diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578–84. doi: 10.2337/dc10-0125. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J, Elliott KJ, Pizzirusso M, Morgello S. Autonomic neuropathy in HIV: A case report and review of potential symptoms in an advanced-stage, HIV cohort. WJA. 2012;2:265–9. doi: 10.4236/wja.2012.23035. [Google Scholar]
- Robinson-Papp J, Byrd D, Rivera Mindt M, Oden NL, Simpson DM, Morgello S, the Manhattan HIV Brain Bank Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch Neurol. 2008;8:1096–101. doi: 10.1001/archneur.65.8.1096. doi: 10.1001/archneur.65.8.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogstad KE, Shah R, Tesfaladet G, Abdullah M, Ahmed-Jushuf I. Cardiovascular autonomic neuropathy in HIV infected patients. Sex Transm Infect. 1999;75:264–7. doi: 10.1136/sti.75.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PJ, Lewis MJ, Sheridan DJ, Henderson AH. Adrenergic hypersensitivity after beta-blocker withdrawal. Br Heart J. 1981;45:637–42. doi: 10.1136/hrt.45.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508–13. doi: 10.1001/archinternmed.2007.106. doi: 10.1001/archinternmed.2007.106. [DOI] [PubMed] [Google Scholar]
- Ruttimann S, Hilti P, Spinas GA, Dubach UC. High frequency of human immunodeficiency virus-associated autonomic neuropathy and more severe involvement in advanced stages of human immunodeficiency virus disease. Arch Intern Med. 1991;151:2441–3. doi: 10.1001/archinte.1991.00400120079013. [DOI] [PubMed] [Google Scholar]
- Sabin CA, Smith CJ, d'Arminio Monforte A, Battegay M, Gabiano C, Galli L, Geelen S, Gibb D, Guiguet M, Judd A, Leport C, Dabis F, Pantazis N, Porter K, Raffi F, Thorne C, Torti C, Walker S, Warszawski J, Wintergerst U, Chene G, Lundgren J. Collaboration of Observational HIV Epidemiological Research Europe (COHERE) Study Group. Response to combination antiretroviral therapy: Variation by age. AIDS. 2008;22:1463–73. doi: 10.1097/QAD.0b013e3282f88d02. doi: 10.1097/QAD.0b013e3282f88d02. [DOI] [PubMed] [Google Scholar]
- Sakhuja A, Goyal A, Jaryal AK, Wig N, Vajpayee M, Kumar A, Deepak KK. Heart rate variability and autonomic function tests in HIV positive individuals in India. Clin Auton Res. 2007;17:193–6. doi: 10.1007/s10286-007-0412-5. doi: 10.1007/s10286-007-0412-5. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L, Clifford DB, ACTG A5117 Study Group HIV neuropathy natural history cohort study: Assessment measures and risk factors. Neurology. 2006;66:1679–87. doi: 10.1212/01.wnl.0000218303.48113.5d. doi: 66/11/1679 [pii]; 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: Mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–65. doi: 10.1523/JNEUROSCI.1247-07.2007. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Nguyen CT, Cox BF, Tarara RP, Capitanio JP, Cole SW. SIV infection decreases sympathetic innervation of primate lymph nodes: The role of neurotrophins. Brain Behav Immun. 2008;22:185–94. doi: 10.1016/j.bbi.2007.07.008. doi: 10.1016/j.bbi.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol. 2006;80:4326–35. doi: 10.1128/JVI.80.9.4326-4335.2006. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez GA, Clark VM, Norell JE, Kottke TE, Callahan MJ, O'Brien PC, Low PA, Dyck PJ. Sudden cardiac death in diabetes mellitus: Risk factors in the Rochester diabetic neuropathy study. JNNP. 2005:240–5. doi: 10.1136/jnnp.2004.039339. doi: 10.1136/jnnp.2004.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O'Brien PC, Low PA. The autonomic symptom profile: A new instrument to assess autonomic symptoms. Neurology. 1999;52:523–8. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- Tiecks FP, Douville C, Byrd S, Lam AM, Newell DW. Evaluation of impaired cerebral autoregulation by the Valsalva maneuver. Stroke. 1996;7:1177–82. doi: 10.1161/01.str.27.7.1177. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: How the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–9. doi: 10.1152/japplphysiol.00470.2003. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Villa A, Foresti V, Confalonieri F. Autonomic neuropathy and prolongation of QT interval in human immunodeficiency virus infection. Clin Auton Res. 1995;5:48–52. doi: 10.1007/BF01845498. [DOI] [PubMed] [Google Scholar]
- Villa A, Foresti V, Confalonieri F. Autonomic nervous system dysfunction associated with HIV infection in intravenous heroin users. AIDS. 1992;6:85–9. doi: 10.1097/00002030-199201000-00011. [DOI] [PubMed] [Google Scholar]
- Zilliox L, Peltier AC, Wren PA, Anderson A, Smith AG, Singleton JR, Feldman EL, Alexander NB, Russell JW. Assessing autonomic dysfunction in early diabetic neuropathy: The survey of autonomic symptoms. Neurology. 2011;76:1099–105. doi: 10.1212/WNL.0b013e3182120147. doi: 10.1212/WNL.0b013e3182120147. [DOI] [PMC free article] [PubMed] [Google Scholar]