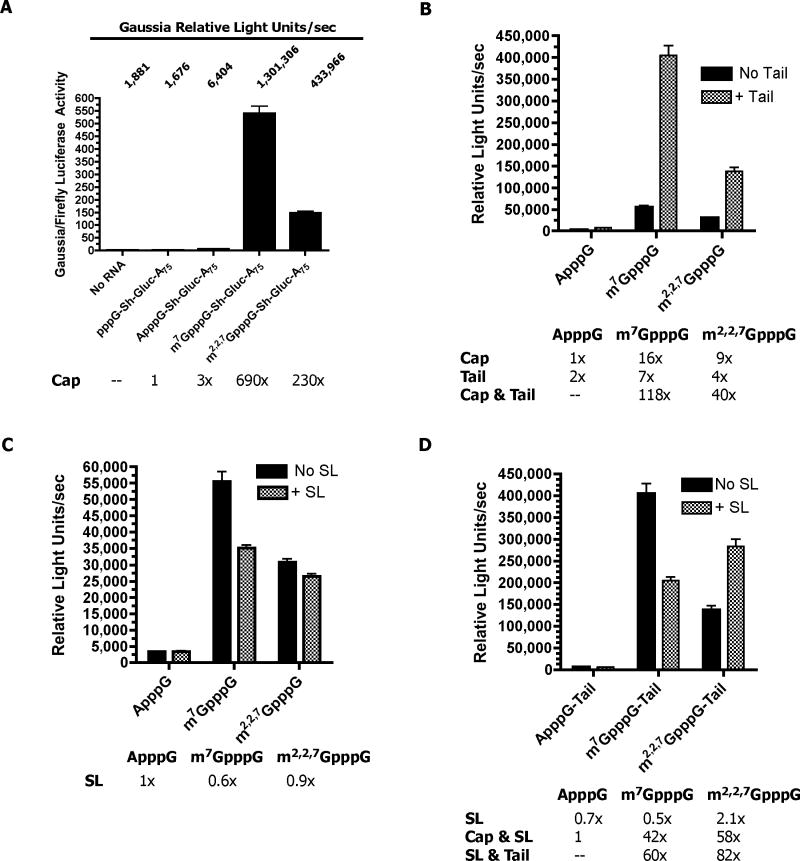
Figure 2. Translation in Ascaris embryos is methyl-guanosine cap-dependent, enhanced by a poly(A) tail, and both a spliced leader and poly(A) tail are required for efficient translation of TMG-capped RNAs.
A) Methyl-guanosine cap dependence of translation. The reporter RNA was Gaussia (Sh Gluc)(5′ UTR = GGUACCGAGCUCGGAUCCAGCCACCAUG) with a 75 nt poly(A) tail (see Suppl. Figure 1). (B) Poly(A) tail enhances translation of methyl-guanosine capped RNAs. The reporter RNA was Ren (5′ UTR = GGUCAUUCCGGUACUGUUAGGCUAGCCACCAUG)(see Suppl. Figure 1) with and without an 85 nt poly(A) tail. C) Effect of spliced leader on translation of different capped RNAs. The reporter RNAs were Ren (no SL) or SL Ren (+ SL) without a poly(A) tail (see Suppl. Figure 1). D) Effect of spliced leader and poly(A) tail on translation of different capped RNAs. The reporter RNAs were Ren (no SL)(5′ UTR = GGUCAUUCCGGUACUGUUAGGCUAGCCACCAUG) or SL Ren (+ SL)(5′ UTR = GGUUUAAUUACCCAAGUUUGAGGGCUAGCCACCAUG) with an 85 nt poly(A) tail (see Suppl. Figure 1). Gaussia (A) or Renilla (B-D) reporter RNAs were biolistically introduced into Ascaris embryos and the luciferase activity measured after 90 minutes. Data illustrate representative experiments and show the mean and standard error derived from triplicate transfections. The Gaussia activity was normalized to a co-transfected Firefly RNA using the Dual-Luciferase Assay.