Abstract
Structural neuroimaging studies have provided evidence of differences in local brain volume between cocaine-dependent and healthy control individuals. While sex differences in aetiology, course and brain dysfunction associated with chronic cocaine abuse have been previously documented, evidence of sex-specific differences in brain volume has not been examined thus far. This study examined sex-related differences in grey matter volume between cocaine-dependent and healthy control subjects using voxel-based morphometry. High-resolution T1 structural scans were obtained from 36 inpatient, treatment-engaged 3-week abstinent cocaine-dependent (CD) individuals. Fifty healthy control subjects were also scanned. Segmentation and registration were performed in SPM8, using New Segment and DARTEL, respectively. The whole-brain statistical analysis was conducted in SPM8 using random field-based cluster-size testing and family-wise error rate correction for multiple comparisons. CD patients were found to have less grey matter volume in anterior prefrontal cortex, including frontopolar and orbitofrontal cortices, and a posterior region surrounding the parietal-occipital sulcus. Female CD patients had less grey matter volume than female controls in left inferior frontal gyrus, insula, superior temporal gyrus and hippocampus. Male CD patients had less grey matter in a superior cortical region that included the precentral gyrus and the mid-cingulate. These sex differences in lower grey matter volume add to the evidence from functional neuroimaging for sex-specific differences in the neurophysiological changes associated with chronic cocaine use.
Keywords: Cocaine dependence, sex, voxel-based morphometry
INTRODUCTION
Animal models of cocaine neurotoxicity (Sharma et al. 2009), and evidence of neuronal injury (Chang et al. 1999) suggest that structural brain abnormalities are associated with cocaine dependence. In structural imaging studies comparing brain volumes in abstinent cocaine-dependent (CD) individuals with healthy controls, lower grey matter volumes (GMVs) have been observed in CD subjects in prefrontal cortex (O'Neill, Cardenas & Meyerhoff 2001; Fein, Di Sclafani & Meyerhoff 2002), orbitofrontal cortex (OFC) (Sim et al. 2007; Ersche et al. 2011), anterior cingulate (Ersche et al. 2011) and premotor cortex (Sim et al. 2007). Lower regional GMVs have also been identified in insula (Ersche et al. 2011), inferior parietal regions (Barros-Loscertales et al. 2011; Ersche et al. 2011), thalamus and cerebellum (Sim et al. 2007). Both smaller (Barros-Loscertales et al. 2011) and larger (Ersche et al. 2011) striatal GMVs have been reported in CD subjects. There is less evidence of white matter differences between CD and control subjects, and the findings are mixed. White matter was significantly reduced in CD subjects relative to controls in the right cerebellum (Sim et al. 2007). However, white matter volume was increased in occipital and posterior parietal cortex in CD subjects relative to controls (O'Neill et al. 2001).
The studies cited here are suggestive of structural abnormalities in individuals with a history of cocaine dependence. These studies, however, included only male participants (Fein et al. 2002; Barros-Loscertales et al., 2011), did not examine sex differences (O'Neill et al. 2001; Ersche et al. 2011) or did not find sex differences (Sim et al. 2007). However, sex-specific patterns of regional brain volume differences between CD and healthy individuals might be expected. In healthy adult humans, sex differences in regional cortical and subcortical relative brain volumes are widely reported (e.g. Luders et al. 2009). These differences are greatest in regions in which the animal homologue shows higher levels of sex-steroid receptors during early brain development (Goldstein et al. 2001), and include regions involved in drug use and addiction, including dopaminergically innervated OFC, anterior cingulate, amygdala and caudate (Volkow, Fowler & Wang 2003). Cocaine-induced dopamine overflow in the striatum was higher in female than male rats (Walker, Ray & Kuhn 2006), a finding of potential importance to sex-specific GMV differences in CD humans, as dopamine neurotoxicity may be involved in cocaine-induced neuronal damage in humans (Little et al. 2009).
Animal models suggest that women may be more vulnerable to substance use initiation, addiction and relapse, and that these effects are mediated by differences in brain organization as well as circulating ovarian hormones (reviewed in Becker & Hu 2008). Sex-related differences have been observed in the aetiology and course of cocaine dependence in clinical samples (McCance-Katz, Carroll & Rounsaville 1999), and in the regional specificity of metabolic changes in CD individuals relative to healthy controls (Chang et al. 1999). Cocaine use imagery was associated with different patterns of regional cerebral blood flow (rCBF) change in CD men compared with CD women, with the men, but not the women exhibiting rCBF increases in the amygdala, left insula and OFC, in response to cocaine cues, and women, but not men showing rCBF increases in the posterior cingulate gyrus (Kilts et al. 2004). In a pair of studies comparing recently abstinent female and male CD subjects to same-sex controls, women demonstrated a blunted rCBF response to limbic stimulation using procaine, including a decrease in OFC, while CD men showed a greater increase relative to controls in temporal and frontal regions, including OFC, but little procaine response in limbic or paralimbic areas (Adinoff et al. 2003). More recently, we have shown robust sex differences in neural responses to stress and drug cues, with abstinent CD women relative to control women showing greater activation in limbic and striatal regions during stress that is not seen in CD men, and CD men relative to control men showing greater activation in these regions during drug cue and neutral relaxing imagery exposure, suggesting clear differences in neural responses to environmental cues known to increase relapse risk (Potenza et al. 2012).
On the basis of the previous literature indicating sex differences in the neuropathology of cocaine addiction, the goal of this study was to examine differences in the pattern of brain volume between recently abstinent CD individuals compared with healthy control volunteers and to assess these differences separately in men and women. Taking advantage of recent advances in image segmentation and registration within the Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology; http://www.fil.ion.ucl.ac. uk/spm), we examined volumetric differences between a sample of abstinent CD men and women and healthy controls using voxel-based morphometry (VBM). On the basis of the prior evidence of cocaine neurotoxicity and lower grey and white matter volume in clinical populations, we predicted lower regional brain volumes, specifically in the frontal brain regions, in abstinent CD subjects relative to controls. In addition, given the known sex-specific differences in brain function associated with chronic cocaine abuse and the importance of the impact of changes in brain regions known to have greater sexual dimorphism on cocaine intake, we expected different patterns of lower regional brain volume in the CD men and women, specifically in the prefrontal and anterior cingulate regions, as well as in limbic-striatal regions, such as the insula, hippocampus, amygdala and posterior cingulate. We also expected that years of cocaine abuse would be associated with decreases in brain volume.
METHOD
Participants
Thirty-six voluntary treatment-seeking individuals (18 men/18 women; ages 25–46) who met DSM-IV Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for current cocaine dependence were admitted to the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center for 4 weeks of inpatient treatment for cocaine dependence and research participation. Current cocaine use was verified by positive urine toxicology screens upon study enrolment. Individuals using opiates and with a past history of opiate dependence were excluded. Alcohol, marijuana and nicotine use prior to admission was included but those requiring medical detoxification from alcohol were excluded. Fifty healthy comparison subjects (28 men/22 women; ages 18–50) were also recruited from the community through local advertisements. Comparison subjects consumed up to 25 alcoholic drinks or less per month (less than 7 drinks/week), had negative urine toxicology screens on admission into the study, and did not meet criteria for current or lifetime abuse or dependence criteria for alcohol or any illicit drug. Individuals taking medications for medical conditions and those on medications for co-morbid psychiatric disorders and those requiring acute medical attention were also excluded. All participants underwent a complete medical examination to insure good physical health. The study was approved by the Human Investigation Committee of Yale University School of Medicine.
Procedures
CD patients were admitted to the Clinical Neuroscience Research Unit, a locked, smoke-free inpatient treatment and research facility with limited access to visitors. Urine and breath tests were conducted at least weekly to ensure continued abstinence. All CD participants admitted into the study participated in specialized substance abuse treatment that included weekly individual therapy provided by psychiatry residents and twice weekly standardized group drug counselling (Mercer et al. 1994) provided by an addiction specialist. The drug treatment was part of the inpatient treatment program that was initiated upon admission and included additional group programming from 9:00 am to 3:30 pm that covered daily life skills and other structured activities. During week 1, patients underwent an initial medical evaluation and provided demographic data and a psychosocial history. During week 2, they were interviewed using the Structured Clinical Interview for DSM-IV (First et al. 1995) to assess psychiatric and substance use diagnoses. Baseline drug-related assessments, including cocaine use for the 90 days preceding admission, were also conducted. Patients underwent a single structural magnetic resonance imaging (MRI) scan between weeks 3 and 4 of cocaine abstinence during their inpatient treatment stay. Healthy control subjects completed demographic, diagnostic and alcohol-related assessments in two to three baseline assessment appointments and underwent a single structural MRI scan after other research appointments. Alcohol breath and urine drug scans were conducted at each appointment to ensure drug free state in the healthy controls.
MRI data acquisition and processing
Data for each subject consisted of a sagittally acquired high-resolution T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) scan: 176 slices, 1 mm3 isotropic voxels, FOV = 256 × 256 mm, data acquisition matrix = 256 × 256, TR = 2530 ms, TE = 3.66 ms, flip angle = 7°.
Processing of MR images
Image segmentation and registration were performed using, respectively, the New Segment and DARTEL registration algorithms incorporated in Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology). New Segment is an extension of the default `Unified Segmentation' algorithm retained from SPM5 (Ashburner & Friston 2005). Unified Segmentation combines tissue classification, bias correction, and image registration steps in an iterative procedure, which results in more accurate tissue classification. New Segment improves the registration model incorporated in Unified Segmentation, and includes additional tissue probability maps to better model cerebrospinal fluid (CSF) and other non-brain voxels, resulting in further improvement to the tissue classification. We used New Segment only to produce the grey matter, white matter, and CSF tissue class images (segmentations) in native space. Nonlinear co-registration of the segmentations was performed in DARTEL (Ashburner 2007), a high-dimensional, diffeomorphic registration algorithm that performed well in a comparison of registration algorithms (Klein et al. 2009). With DARTEL, we expected to increase registration accuracy, thereby increasing sensitivity and improving localization in comparisons of the CD and healthy control groups.
As a first processing step, the native-space images were aligned with the plane of the anterior and posterior commissures and the image space origin manually set to the anterior commissure. Default settings were used for segmentation. No skull stripping was applied prior to segmentation. As manual segmentation is considered the `gold standard' for evaluating the quality of automated tissue classification, the resulting segmentations were validated visually. Particular attention was given to the thickness of the cortical surface, which was compared visually with each subject's native space image, and also compared with a published report of cortical thickness (Fischl & Dale 2000). Segmentation demonstrated appropriate face validity for all images.
As in previous whole-brain VBM studies (for recent examples, cf. Hogan et al., 2001; Colloby et al., 2011), we calculated total intracranial volume (TICV) estimates for inclusion as a covariate in our statistical model (see Data Analysis) by summing the grey matter, white matter and CSF segments to comprise TICV (cf. Pfefferbaum et al. 1994) for each subject. TICV is considered a stable measure of premorbid brain volume (e.g. Jenkins et al. 2000). As expected, we found no differences between CD and control subjects in TICV, and greater male than female TICV (Table 1).
Table 1.
Total intracranial volume (TICV) estimates in millilitres.
| CD | Healthy control | Total | |
|---|---|---|---|
| Females | 1411.15 | 1393.63 | 1401.52* |
| Males | 1556.13 | 1580.57 | 1571.00* |
| Total | 1483.64** | 1498.31** | 1492.17 |
Significant greater TICV in men relative to women (F(1, 82) = 41.95, P < 0.001).
Note: No differences in TICV between cocaine-dependent (CD) and control subjects (F(1, 82) = 0.018, P = 0.893).
The rigid-body aligned grey matter, white matter and CSF segmentations (1.5 mm^3 voxels) for each subject were registered in DARTEL using the default parameter settings. DARTEL performs a series of template creation and registration steps, improving the registration of the data set with successive iterations. The final template is an average of the DARTEL registered data, and the images are registered to this space. Therefore, in an additional step, the DARTEL-registered data were affine-transformed into MNI space, modulated, and smoothed using a 6 mm Gaussian filter. Modulation rescales voxel values to reflect volume differences between images throughout the brain. Gaussian smoothing reduces the effects of residual misregistration on potential group differences and reduces departures from normality that may occur at some voxels. Separate analyses were conducted using the grey matter and white matter segmentations.
Data analysis
Statistical parametric maps were created in SPM8 to perform between-group comparisons using the normalized, modulated and smoothed grey matter tissue segmentations output by DARTEL. A general linear model was created with diagnostic group (CD vs. healthy control), and sex as factors of interest, and voxel-based whole-brain GMV as the dependent measure. Covariates included subject age and an estimate of TICV calculated by voxel-wise summation in a MATLAB script (2008a; The Mathworks, Inc., Natick, MA; Ged Ridgway, http://www.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m, accessed 17 September 2010) of the native space grey, white and CSF segmentations for each subject. A whole-brain VBM analysis was then conducted comparing GMV differences adjusted for age effects, as well as the influence of individual differences in global brain size. An identical analysis was performed using the white matter tissue segmentations.
Whole brain analysis
The whole-brain statistical analysis was conducted in SPM8 using random field-based cluster-size testing and family-wise error rate (FWE) correction for multiple comparisons. The cluster size test increases sensitivity, relative to voxel-based tests, for spatially extended signals, and low thresholds increase the power of these tests for the signals of large spatial extent characteristic of spatially smooth data (Friston et al. 1996). Clusters were formed from contiguous voxels exceeding an uncorrected one-tailed threshold of P < 0.025. The FWE corrected threshold for significant cluster size was set at one-tailed P < 0.025. All one-tailed tests of group and sex differences were performed in both directions for an overall P < 0.05 significance threshold.
Specific anatomical regions were delineated from MNI space coordinates within the significant clusters identified in SPM outputs, using the Automated Anatomy Library (Tzourio-Mazoyer et al. 2002) within the WFU Pickatlas (Maldjian et al. 2003). Anatomical regions were confirmed and Brodmann areas (BAs) determined using the Talairach Atlas (Talairach & Tournoux 1988) after conversion to Talairach coordinates. MNI to Talairach conversion was performed using the Nonlinear Yale MNI to Talairach Conversion Algorithm (Lacadie et al. 2008).
RESULTS
Sample demographic, substance use and psychiatric history
There were no differences between the CD and the healthy control groups on sex and race. However, the CD patients were older, with a lower IQ, and included more smokers than the control group. The amount of alcohol used in the 30 days prior to treatment and the number of days of alcohol use during the same 30 days was significantly greater in abstinent CD subjects than healthy controls (Table 2).
Table 2.
Demographics and clinical characteristics of cocaine-dependent (CD) and healthy control subjects.
|
CD patients (n = 36) |
Healthy comparison subjects (n = 50) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Male
|
Female
|
Male
|
Female
|
|||||
| n | % | n | % | n | % | n | % | |
| Sex | 18 | 50.0 | 18 | 50.0 | 28 | 56.0 | 22 | 44.0 |
| Caucasian | 8 | 22.2 | 7 | 19.4 | 18 | 36.0 | 10 | 20.0 |
| African-American | 9 | 25.0 | 11 | 30.6 | 4 | 8.0 | 9 | 18.0 |
| Hispanic | 1 | 2.8 | 0 | 0.0 | 4 | 8.0 | 3 | 6.0 |
| Asian | 0 | 0.0 | 0 | 0.0 | 2 | 4.0 | 0 | 0.0 |
| Lifetime prevalence of alcohol dependence | 8 | 44.4 | 4 | 22.2 | – | – | – | – |
| Number of smokers* | 14 | 77.8 | 15 | 83.3 | 4 | 14.3 | 3 | 10.7 |
|
| ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||||
| Age** (years) | 38.22 | 5.38 | 36.67 | 5.63 | 30.86 | 9.74 | 31.50 | 8.27 |
| Estimated IQ*** | 110.28 | 11.17 | 105.00 | 10.32 | 115.46 | 7.56 | 115.14 | 6.71 |
| Recent cocaine use | ||||||||
| Days cocaine use last 90 days | 46.78 | 23.50 | 45.72 | 25.12 | – | – | – | – |
| Total cocaine use last 90 days | 47.50 | 42.71 | 48.55 | 55.28 | – | – | – | – |
| Mean cocaine use per use last 90 days | 1.22 | 1.50 | 1.11 | 0.76 | – | – | – | – |
| Years of cocaine use | 11.53 | 6.47 | 8.89 | 6.52 | – | – | – | – |
| Recent alcohol use | ||||||||
| Days of alcohol use past 30 days+/+++ | 8.11 | 8.07 | 12.61 | 8.32 | 5.54 | 6.29 | 2.32 | 3.05 |
| Amount of alcohol use past 30 days (drinks/mth)++ | 87.43 | 117.45 | 129.14 | 111.97 | 9.95 | 9.04 | 3.77 | 6.53 |
Cocaine subjects > comparison subjects, chi-square (1) = 38.10, P < 0.001.
Cocaine subjects > comparison subjects, t(84) = 3.72, P < 0.001.
Cocaine subjects < comparison subjects, t(84) = −3.94, P < 0.001.
Cocaine subjects > comparison subjects, t(84) = 4.21, P < 0.001.
Cocaine subjects > comparison subjects, t(65) = 5.21, P < 0.001.
Healthy comparison men > healthy comparison women, t(48) = 2.20, P = 0.033.
Group and sex differences in VBM analysis
CD patients were found to have two clusters of significantly less GMV than controls. The frontal cluster (Fig. 1a–c) included frontopolar (BA10) and OFC (BA 11 and 47) bilaterally, and right dorsolateral cortex (BA 46/45) (FWE P < 0.001, cluster size = 8851 voxels). The posterior cluster (Fig. 1d–f) in parietal, occipital and temporal cortex included the posterior cingulate (BA29/30), precuneus (BA18/31) and cuneus regions (BA18), middle occipital (BA19), inferior occipital (BA18/19), and lingual gyri (BA17/18), and the posterior middle (BA22/39) and inferior temporal lobe (BA19/37; FWE P < 0.001, cluster size = 7632 voxels). A lateral region in the left cerebellum (Fig. 2a–c) was found to be significantly larger in CD subjects than healthy controls (FWE P = 0.018; cluster size = 3346 voxels). No significant differences between groups were observed in the comparison of white matter volumes.
Figure 1.
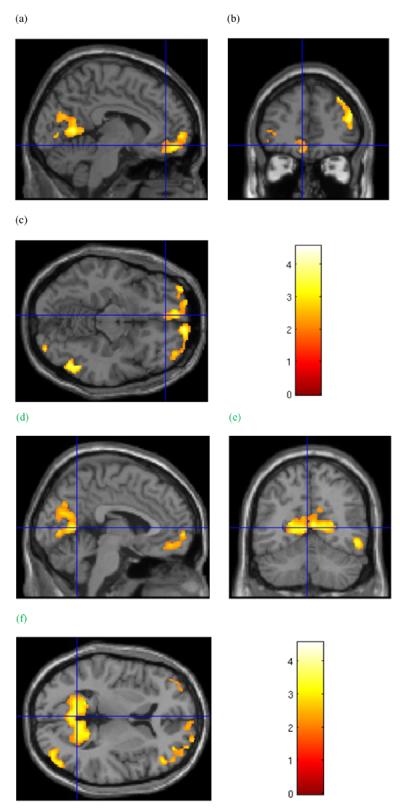
Group grey matter volume effects. Cluster in the frontal lobes of significantly lower grey matter volume in cocaine-dependent subjects relative to healthy controls. Cursor location: X=−6.0, Y=43.5, Z=−12.0, left medial orbitofrontal cortex (a and c). Also shown: left cuneus and precuneus region (a) in the posterior cortical cluster, and right dorsolateral prefrontal cortex (b). d–f: Cluster of significantly lower grey matter volume in cocaine-dependent subjects relative to healthy controls that includes the precuneus and cuneus regions surrounding the parietal-occipital sulcus. Cursor location: X=−3.0, Y=−58.5, Z = 6.0. Colour bar indicates voxel-wise t-statistics
Figure 2.
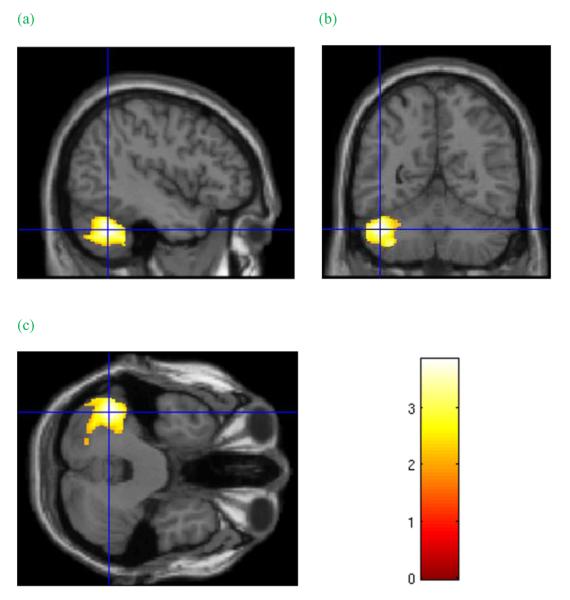
Group grey matter volume effects. Cluster in the left cerebellum of significantly greater grey matter volume in cocaine-dependent subjects relative to healthy controls. Cursor location: X=−43.5, Y=−55.5, Z=−34.5. Colour bar indicates voxel-wise t-statistics
Female subjects had lower GMV than male subjects, bilaterally, in the cerebellum, and posterior cingulate gyrus (BA23/30; FWE P = 0.003, cluster size = 4425 voxels). Female subjects had greater GMV than male subjects in the left superior temporal gyrus (BA38), and left insular, orbital, inferior, middle, and superior frontal cortices (BA10, 44, 45, 47; FWE P = 0.014, cluster size = 3482 voxels) (see Table 3). No significant sex differences were observed in the comparison of white matter volumes. In addition, no sex differences in either grey or white matter volumes that met the FWE corrected threshold were found within either cocaine (i.e., cocaine men versus women) or the healthy control (healthy men versus women) groups.
Table 3.
Grey matter volume clusters of significant difference between cocaine-dependent (CD) patients and healthy controls (HC): regions within significant clusters from the Talairach Atlasa,b.
| Cluster | FWE Cluster P-value | Voxels in cluster | Cluster volume (ml) | Brodmann areab |
|---|---|---|---|---|
| Group effect | ||||
| CD < HC | ||||
| Frontopolar and orbitofrontal cortex, gyrus rectus; inferolateral cortex; r. dorsolateral prefrontal cortex | P < 0.001 | 7632 | 25.76 | 9, 10, 11, 45, 46, 47 |
| Lingual gyrus, middle and inferior occipital gyrus, cuneus; precuneus, posterior cingulate gyrus; r. middle and inferior temporal gyrus | P < 0.001 | 8851 | 29.87 | 17, 18, 19, 22, 23, 29, 30, 31, 37 39 |
| CD > HC | ||||
| 1. cerebellum, lateral lobule | P = 0.018 | 3346 | 11.29 | – |
| Sex effect | ||||
| Females < males | ||||
| cerebellum, posterior cingulate gyrus | P = 0.003 | 4425 | 14.93 | 23, 30 |
| Females > males | ||||
| left superior temporal gyrus; left insula; left orbitofrontal, inferior, middle and superior frontal gyri | P = 0.014 | 3482 | 11.75 | 10, 38, 44, 45, 47 |
| Females: CD < HC | ||||
| 1. Inferior frontal gyrus; 1. Insula; 1. Superior temporal gyrus, transverse temporal gyrus; 1. Hippocampus | P = 0.004 | 4258 | 14.37 | 22, 41, 42, 47 |
| r. Lingual gyrus, middle and inferior occipital gyrus; r. angular gyrus, supramarginal gyrus; r. middle and inferior temporal gyrus | P = 0.011 | 3653 | 12.33 | 18, 19, 37, 39, 40 |
| Females CD > HC | ||||
| r. cerebellum, lateral lobule | P = 0.003 | 4474 | 15.10 | – |
| 1. cerebellum, lateral lobule | P = 0.023 | 3219 | 10.86 | – |
| Males: CD < HC | ||||
| Superior frontal, precentral and paracentral gyrus, midcingulate gyrus | P = 0.021 | 3260 | 11.00 | 4, 6 |
Lacadie et al., 2008.
Talairach and Tournoux, 1988.
Sex effects in VBM analysis
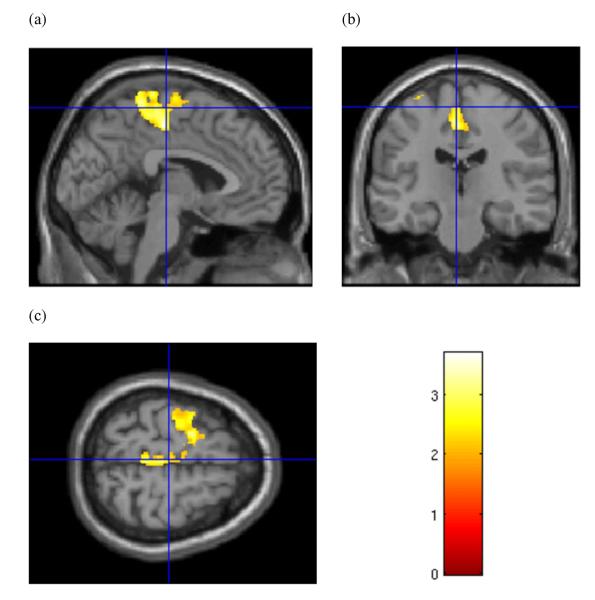
We observed sex-specific volume differences between the CD and control subjects. CD women were found to have two clusters of significantly less GMV than female controls: a posterior cluster in the right hemisphere (Fig. 3d–f), centred around the inferior (BA18) and middle (BA19/37/39) occipital gyri, extending into the middle and inferior temporal gyri (BA37), and the inferior parietal lobe, including the angular and supramarginal gyri (BA39/40; FWE P = 0.011, cluster size = 3653 voxels); and a left hemisphere cluster (Fig. 3a–c) centred around insular cortex, extending through the superior temporal gyrus (BA22/42) and medially to include the hippocampus, as well as anteriorly into the inferior frontal gyrus (BA47; FWE P = 0.004, cluster size = 4258 voxels). CD females were found to have larger GMVs bilaterally in the cerebellum (left: FWE P = 0.023, cluster size = 3219; right: FWE P = 0.003, cluster size 4474) (see Fig. 4). No significant group differences were observed in the comparison of female white matter volumes.
Figure 3.
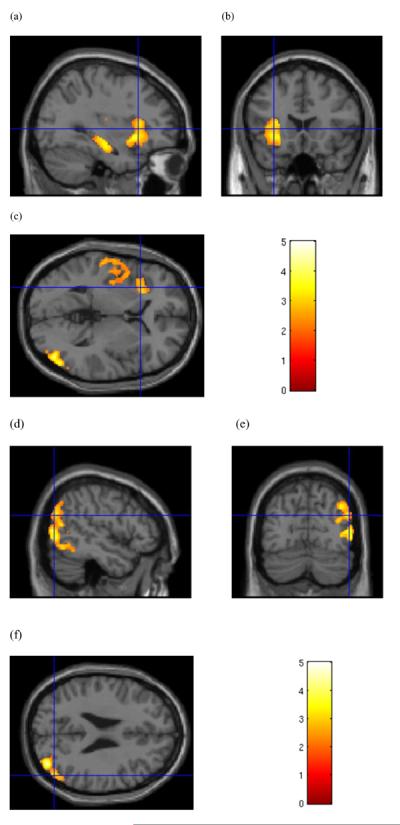
Sex-specific grey matter volume effects in female subjects. (a–c) Cluster of significantly lower grey matter volume in cocaine-dependent (CD) female subjects relative to healthy controls. Cursor location: X=−31.5, Y = 19.5, Z = 3.0, left anterior insula (b).Also shown: left hippocampus and left superior temporal gyrus (a), and right middle occipital gyrus (c) in the posterior cortical cluster from the analysis of female subjects. (d–f) Cluster of significantly lower grey matter volume in CD female subjects relative to healthy controls along the right temporal-occipital boundary. Cursor location: X = 51.0, Y=−73.5, Z = 25.5. Colour bar indicates voxel-wise t-statistics
Figure 4.
Sex-specific grey matter volume effects in female subjects. Cluster of significantly greater grey matter volume in cocaine-dependent female subjects relative to female healthy controls in the cerebellum, bilaterally. Cursor location: X = 37.5, Y=−52.5, Z=−27.0, right cerebellum. Colour bar indicates voxel-wise t-statistics
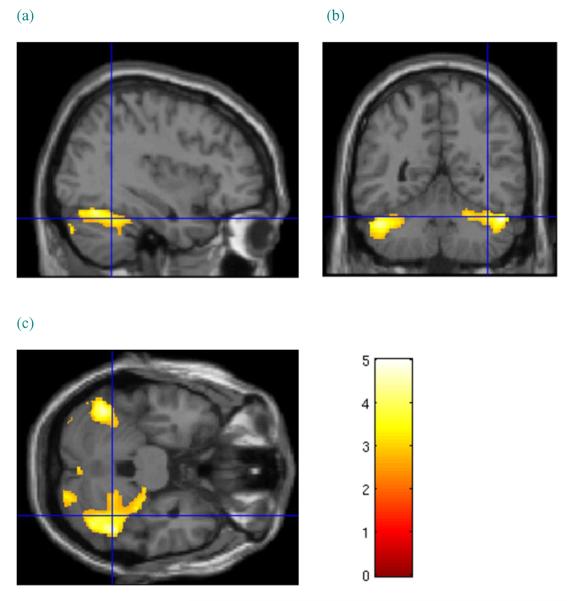
CD men were found to have significantly less GMV in a cluster that included superior cortical areas (FWE P = 0.021, cluster size = 3260 voxels), including the midcingulate cortex and supplementary motor area (BA31), superior and middle frontal gyri, medial frontal gyrus, paracentral lobule, and precentral and postcentral gyri, bilaterally (BA4/6; Fig. 5). No clusters were found for which volumes in the CD men were significantly larger than in the control males. No significant group differences were observed in the comparison of male white matter volumes.
Figure 5.
Sex-specific grey matter volume effects in male subjects. Cluster of significantly lower grey matter volume in cocaine-dependent male subjects relative to healthy controls. Cursor location: X=−3.0, Y=−21.0, Z = 63.0. The sagittal view (a) most clearly shows left supplementary motor area, paracentral lobule and midcingulum. Colour bar indicates voxel-wise t-statistics
As we have previously shown smaller GMVs in abstinent alcohol dependent individuals compared with healthy controls (Rando et al. 2011), we conducted follow-up analyses to assess the possible confounding association of alcohol use with lower GMV in our CD sample. A whole brain analysis was conducted with GMV as the dependent measure, and the amount of alcohol use in the 30 days before treatment, age, sex and TICV as covariates. Using an FWE corrected threshold for significant cluster size set at one-tailed P < 0.025, we found a significant negative association between GMV and amount of alcohol in the left cerebellum (FWE P = 0.019, 3698 voxels). We also found a significant negative association between GMV and the number of days of alcohol use in the 30 days before treatment, with age, sex and TICV as additional covariates, in a region that included the parahippocampal gyrus, fusiform gyrus and lingual gyrus (BA 18, 19, 27, 30, 36, 37; FWE P = 0.013, 4025 voxels). We also included lifetime years of alcohol use as a covariate in the original analyses, and the current results comparing CD patients to controls and the results presented in Figures 1–4 and Table 3 were unchanged. Furthermore, extracted GMV within the significant clusters of regions showing differences between groups (Fig. 1) and within sex (Figs 2,3) were also not correlated with alcohol use measures (r's ranging from −0.268 to 0.210, P > 0.05). Thus, these findings suggest that the specific GMV differences between CD men and women, and controls reported here were not due to recent or lifetime levels of alcohol consumption. Although the CD group had lower IQ than healthy subjects, IQ in this group was not correlated with the significant GMV clusters that were different from controls (r's ranging from −0.082 to 0.212, P > 0.05), nor was it correlated with years of cocaine use (r = 0.073, P = 0.677) or amount of cocaine use (r = 0.11, P = 0.539).
Correlation of regional GMV differences with baseline cocaine use measures
To examine whether GMVs in the clusters of significantly lower GMV in CD subjects were associated with cocaine use, a region of interest (ROI) analysis was conducted. Each significant cluster was converted to a binary mask using MarsBar (http://marsbar.sourceforge.net). These masks and the ImCalc function in SPM8 were used to extract the modulated grey matter voxels to create the ROI for each subject within each masked region. A MATLAB script (2008a; The Mathworks, Inc.) summed the voxels for each subject within each ROI to calculate total GMVs (in millilitres) for each significant cluster. Pearson product moment correlations were conducted between these significant ROIs and clinical variables of cocaine use. Due to modest sample size for each gender, correlations were conducted for the whole group for all significant clusters. A significant negative correlation was found between volume in the midcingulate/superior frontal gyrus cluster and the number of years of cocaine use (r = −0.336, P = 0.048). Greater number of years of cocaine use was associated with lower GMV in this region. Although age was correlated with years of cocaine use (r = 0.426, P = 0.011), it was not associated with GMVs in the significant clusters in the current sample of 21- to 50-year old participants (r's ranging from −0.304 to −0.109, P's ranging from 0.534–0.075, P > 0.05), and we did not include it as a covariate when correlating years of cocaine use with GMVs in the significant clusters. No other cocaine use measures, such as recent cocaine use, were associated with GMV changes in the significant clusters.
DISCUSSION
In this study, compared with healthy controls, abstinent CD subjects were found to have smaller GMV in a number of cortical regions and larger GMV in the left cerebellum. Importantly, this study is the first to examine and report sex-specific patterns of GMV differences between CD and healthy comparison subjects. We found lower GMV in the left inferior frontal gyrus, insula, superior temporal gyrus, and hippocampus in female CD subjects, as well as in temporal, parietal, and occipital cortices, compared to female controls. Male CD subjects were found to have lower GMV in a large area of the superior frontal cortex compared with male controls.
As previous grey matter studies of cocaine dependence have included male or predominantly male subjects, comparisons with the current study must be evaluated cautiously. Our results confirm the frequent finding of lower grey-matter volume in CD subjects in OFC (Sim et al. 2007; Alia-Klein et al. 2011; Ersche et al. 2011; Moreno-López et al., 2012) and dorsolateral and inferolateral frontal cortex (O'Neill et al. 2001; Fein et al. 2002; Sim et al. 2007; Alia-Klein et al. 2011; Moreno-López et al., 2012). Other groups have found lower left insula (Ersche et al. 2011) and left hippocampus volume (Moreno-López et al., 2012), findings specific to our female subjects, lower GMV in posterior medial frontal cortex (Fein et al. 2002; Sim et al. 2007; Ersche et al. 2011), a finding specific to our male subjects, and at the temporal-parietal-occipital boundary (Alia-Klein et al. 2011; Ersche et al. 2011). In addition, we found larger cerebellar GMV, in agreement with some (Ersche et al. 2011) but not all previous studies (Sim et al. 2007; Ersche et al. 2011). We did not replicate findings of lower GMV in cocaine subjects in anterior cingulate cortex (Ersche et al. 2011; Moreno-López et al., 2012), or GMV differences in the striatum (Barros-Loscertales et al. 2011; Ersche et al. 2011; Moreno-López et al., 2012).
We found sex-specific differences in limbic and paralimbic regions, with only CD women showing lower GMV in the left hippocampus and insula. The hippocampus (Fanselow & Dong 2010) and insula (e.g. Phan et al. 2005) are important to stress and emotion regulation, and stress has been established as a risk factor for drug abuse and relapse (Sinha 2008). Functional neuroimaging evidence exists for sex-specific differences in the cocaine-related changes in physiology in the corticostriatal-limbic circuits involved in emotion, stress and motivation, including the hippocampus (Potenza et al. 2012) and insula (Adinoff et al. 2003; Potenza et al. 2012). In Potenza et al. (2012), while limbic hyperactivations, including the insula and hippocampus, involved similar regions in CD men and women, this increased activation was seen in response to different cue contexts. Greater activations were found in CD men in response to cocaine cues, while CD women activated similar limbic areas in response to cues for stressful situations. Lower GMV in the insula and hippocampus may contribute to previously reported stress-induced hyperresponsivity in the neural circuitry involved in emotion, stress and drug addiction in CD women. If lower GMV in these regions confers greater risk of stress-induced cocaine use, CD women may benefit from treatment strategies that target stress pathophysiology to improve both function and brain atrophy.
It is also interesting to consider the insula finding in terms of its involvement in emotional awareness. Damage to the insula has been associated with the loss of emotional self-awareness and self-conscious behaviour and insight into the consequences of behaviour (Goldstein et al. 2009b). The insula has also been implicated in the experience of craving (Naqvi & Bechara 2009). While Potenza et al. (2012) found stress cue-related neural activations in CD women, these activations did not correlate with subjective craving. The absence of these correlations in the CD women suggests that they may have difficulty using internal signals to regulate behaviour. It is possible that lower insula volume may disrupt the awareness of these interoceptive signals. Treatment of CD women may therefore benefit from strategies that promote the conscious use of contextual cues to improve awareness of the need for emotion regulation and avoidance of cocaine use.
Lower GMV in the superior frontal cortex, including the precentral gyrus, was found only in the CD men. fMRI using a stop signal task suggests that precentral gyrus activation is important to response inhibition (Li et al. 2006). Reward reduced activation in the precentral gyrus during stop signal task performance and increased stop signal reaction time (SSRT), indicating that response inhibition was more difficult when reward was present. Cocaine cues, with their rewarding associations, led to hyperactivations in the corticostriatal-limbic emotion and reward circuitry in CD men (Potenza et al. 2012). Activation of the reward circuitry may diminish activation of the precentral gyrus at a time when greater activation is needed to implement cognitive control. Lower GMV in the precentral gyrus may compromise this control, leaving CD men at greater risk of impulsive decision to use cocaine. Cognitive behavioural strategies that promote alternative responses to cocaine use when exposed to drug cues may be helpful to CD men.
Our results confirm the frequent finding of lower GMV in the OFC. The significant prefrontal cluster in this study reveals lower GMV in frontopolar cortex and both medial and lateral OFC in the CD subjects. While lateral OFC has been associated with sensitivity to punishment, medial OFC has been associated with sensitivity to reward (see Rolls, 2004 for a review), and Ersche et al. (2011) found a significant correlation between cocaine-related compulsivity in a subregion of the medial OFC that overlaps with the OFC-inclusive prefrontal cluster in the current study. Sex-specific effects in the OFC might be expected, given that hypoperfusion has been found in the lateral OFC in CD men and the medial OFC in women (Adinoff et al. 2006), but, like other researchers, we did not find sex-specific effects in the OFC. If the hypoperfusion effects observed by Adinoff and colleagues are associated with sex-specific GMV effects, larger samples of men and women may be necessary to localize these effects. Nonetheless, our results are consistent with cocaine-related dysregulation in OFC, a region in which disrupted function has been linked to reward dysregulation and the development and maintenance of addictive behaviours (Volkow et al. 2003). Thus, the current finding that CD subjects had lower OFC GMV suggests that it could be a contributing factor to the dysregulation of OFC-based functions in cocaine abuse.
We found lower GMV in the CD group in a large posterior region. This region included the precuneus and posterior cingulate cortex, regions included in the `default mode' brain network (DMN) that shows functional connectivity when the brain is in a resting state, and is deactivated during a cognitive task (Grecius et al. 2003). Recent work indicates the importance of the DMN in a variety of neuropsychiatric disorders (Broyd et al. 2009). Unlike controls, CD subjects failed to show error-related changes in activations in the DMN (Bednarski et al. 2011). The current finding of lower GMV in the precuneus and posterior cingulate is consistent with this previous evidence of dysregulation in the DMN in cocaine dependence.
As noted earlier, this study and others have identified both smaller and larger regions of cerebellar GMV in CD subjects. The human cerebellum is sensitive to cocaine administration (Volkow et al. 2003), and neuroadaptations have been found in rats administered cocaine (Dietrich et al. 2007). However, the significance to cocaine dependence of smaller versus larger cerebellar volume is unclear. Obsessive compulsive disorder has been associated with larger cerebellar volume (Pujol et al. 2004), and higher compulsivity in cocaine addicts has been associated with cerebellar activation (Ersche et al. 2010). Larger cerebellar GMVs may contribute to compulsive behaviours, including cocaine use. It may be speculated that greater cerebellar volume may also be a marker for a neurobiological vulnerability to substance dependence while the lower volumes sometimes observed may be an effect of substance use (Hill et al. 2011). We found no significant association between cerebellar GMV and cocaine use measures, suggesting that larger cerebellar GMV volume in CD subjects relative to controls is not related to chronic use of cocaine, pointing to the possibility that it could be a vulnerability marker for cocaine dependence. We cannot, however, rule out the possibility that cocaine dependence contributes to lower cerebellar GMV, as observed by Ersche and colleagues.
LIMITATIONS AND CONCLUSIONS
This study detected GMV differences in a number of regions when voxel-based whole-brain analyses were conducted to compare CD and healthy control samples. However, we may have failed to detect differences, especially in smaller anatomical structures, because of residual misregistration. Nonetheless, the newer segmentation and registration algorithms in SPM8 were expected to greatly improve registration accuracy. Cluster-size testing was also expected to increase sensitivity, but restricted our ability to localize group volume differences to the region bounded by each cluster of significant difference. However, an analysis based on cluster extent is appropriate for smooth, spatially extended signals like those present in smoothed structural imaging data, and taken together with the improvement in sensitivity, makes the cluster-size test appropriate and meaningful for this analysis. Finally, the modest sample size may have reduced the power to detect differences, particularly in the ability to detect sex-specific effects within each group.
Most CD subjects smoked, and an association between cigarette smoking and GMVs in substance-dependent subjects has been found (Durazzo et al. 2007). CD subjects also used more alcohol than controls, and twelve CD subjects met criteria for alcohol dependence. Alcohol and nicotine use and abuse are highly co-morbid with cocaine use disorders, and most controls are non-smokers and drink significantly less than CD individuals. Thus, the current study is limited in identifying the GMV effects of only cocaine abuse, and it is likely that the current results represent combined effects of use of these substances. Larger samples of CD and healthy comparison men and women better matched on alcohol and nicotine use are needed. Finally, IQ was significantly lower in the CD sample. While lower IQ is highly associated with the phenomenology of substance use disorders and commonly observed during early abstinence from cocaine, it is important to note that IQ was not correlated with GMV in the CD group. Thus, the findings remain limited by the inability to account for any premorbid differences in IQ between CD and control subjects. Despite these limitations, the current findings support the growing body of evidence of GMV changes associated with chronic cocaine abuse and also indicate sex-specific differences in the neurophysiological changes associated with chronic cocaine use.
Acknowledgements
This research was supported by the National Institutes of Health grants P50-DA016556 (RS) and the NIH Roadmap Fund for Medical Research grants: UL1-DE019586 (RS), PL1-DA024859 (RS) and UL1-RR024139 (Yale CTSA) as well as the Department of Mental Health and Addiction Services of the State of Connecticut. We thank the staff at the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center and the Hospital Research Unit of the Yale Center for Clinical Investigation for their assistance in completing this study.
Footnotes
Disclosures/Conflict of Interest
All of the authors report that they have no conflicts of interest over the past 5 years to report as related to the subject of the report. Dr. Guarnaccia has served on the speaker panel and as a consultant for Biogen, Inc., Teva Pharmaceuticals, Accorda Pharmaceuticals, Pfizer, Inc., Serono, Inc., Bayer Pharmaceuticals and Abbott Pharmaceuticals. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics.
Authors Contribution
KR and RS designed the study. KT and JH oversaw subject recruitment, clinical characterization and abstinence and recovery of CD patients during the inpatient phase. JBG oversaw physical health and clinical characterization of the control participants, as well as provided consultation on neuroanatomy. KR and RS interpreted the data and wrote the paper.
References
- Adinoff B, Devous MD, Best SE, Harris TS, Chandker P, Frock SD, Williams MJ. Regional cerebral blood flow in female cocaine-addicted subjects following limbic activation. Drug Alcohol Depend. 2003;71:255–268. doi: 10.1016/s0376-8716(03)00138-8. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD., Sr Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend Med. 2006;3:206–222. doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KF. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarski SR, Zhang S, Hong KI, Sinha R, Rounsaville BJ, Li CS. Deficits in default mode network activity preceding error in cocaine dependent individuals. Drug Alcohol Depend. 2011;119:e51–e57. doi: 10.1016/j.drugalcdep.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolic changes in the frontal lobes of abstinent cocaine user. Am J Psychiatry. 1999;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- Colloby SJ, Firbank MJ, Vasudev A, Parry SW, Thomas AJ, O'Brien JT. Cortical thickness and VBM-DARTEL in late-life depression. J Affect Disord. 2011;133:158–164. doi: 10.1016/j.jad.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Dietrich JB, Arpin-Bott MP, Kao D, Dirrig-Grosch S, Aunis D, Zwiller J. Cocaine induces the expression of homer 1b/c, homer 3a/b, and hsp 27 proteins in rat cerebellum. Synapse. 2007;61:587–594. doi: 10.1002/syn.20412. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Simon Jones P, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Bullmore ET, Craig KJ, Shabbir SS, Abbott S, Müller U, Ooi C, Suckling J, Barnes A, Sahakian BJ, Merlo-Pich EV, Robbins TW. Influence of compulsivity of drug abuse on dopaminergic modulation of attentional bias in stimulant dependence. Arch Gen Psychiatry. 2010;67:632–644. doi: 10.1001/archgenpsychiatry.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Patient Edition American Psychiatric Press, Inc.; Washington, DC: 1995. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline J-B, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009b;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, McDermott M, Zezza N, Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: association with allelic variation in GABRA2 and BDNF. Psychiatry Res. 2011;194:304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Murray AD, Ahearn TS, Deary IJ, Whalley LJ. Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex. 2001;47:441–450. doi: 10.1016/j.cortex.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Jenkins R, Fox NC, Rossor AM, Harvey RJ, Rossor MN. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Arch Neurol. 2000;57:220–224. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteran T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-SR, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Ramssen E, Welchko R, Volber V, Roland CJ, Cassin B. Decreased brain dopamine cell numbers in human cocaine users. Psychatry Res. 2009;168:173–180. doi: 10.1016/j.psychres.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 2009;29:14265–14270. doi: 10.1523/JNEUROSCI.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers- implications for treatment and prognosis. Am J Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- Mercer D, Carpenter G, Daley D, Patterson C, Volpicelli J. Addiction Recovery Manual. Vol. 2. Treatment Research Unit, University of Pennsylvania; Philadelphia, PA: 1994. [Google Scholar]
- Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, Verdejo-García A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong K, Lacadie CM, Fulbright RK, Li C-SR, Bergquist KL, Sinha R. Neural correlates of stress-induced and cue-induced craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Rando K, Hong K, Bhagwagar Z, Li C-SR, Bergquist K, Guarnaccia J, Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: a prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Muresanu DF, Sharma A, Patnaik R. Cocaine-induced breakdown of the blood-brain barrier and neurotoxicity. Int Rev Neurobiol. 2009;88:297–334. doi: 10.1016/S0074-7742(09)88011-2. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neuro-chemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]