Abstract
Although the pathophysiology of immunoglobulin E (IgE)-mediated allergic rhinoconjunctivitis and bronchial asthma is rather well established, the role of allergy in atopic eczema (AE) is still controversial. By a technique called atopy patch test, aeroallergens like house dust mite, animal dander, or pollen were proven as relevant trigger factors in a subgroup of patients with AE. The atopy patch test is an epicutaneous patch test with such allergens known to elicit IgE-mediated reactions, and used for the evaluation of eczematous skin reactions. In a series of single-center and multicenter studies, a method was developed, standardized, and compared with other diagnostic techniques (radioallergosorbent test, skin prick test) in AE patients. With regard to clinical history, the most specific results were obtained with the atopy patch test (allergen-dependent, 69%-92%), whereas sensitivity was higher for skin prick test (range, 69%-82%) and specific IgE (range, 65%-94%). The characterization of a patient subgroup with relevant IgE-mediated allergy may lead to more efficient avoidance and eventually even specific immunotherapy strategies in the management of AE.
Keywords: IgE, diagnostic techniques, atopy patch test, atopic eczema
Atopic eczema (AE, atopic dermatitis, AE/dermatitis syndrome) is a clinically well-defined inflammatory, chronically relapsing, highly pruritic skin disease with a typically age-related distribution and morphology[1-3] and a prevalence of 2% to 10% in the population [1,4,5]. Elevated immunoglobulin E (IgE) production, especially against aeroallergens and food allergens, and/or altered unspecific reactivity are frequent findings in patients with AE and concomitant respiratory atopic diseases [6,7]. As a multifactorial disease with a genetic background, AE has a large number of individually different trigger factors [8-11].
The deterioration of AE skin lesions in some patients after contact with certain IgE-inducing allergens like house dust mite, pollen, or animal dander is an old clinical observation. Consequently, allergen avoidance strategies have been used to improve the course of AE in some studies [12-17].
The inflammatory infiltrate of AE lesions consists to a large proportion of CD4+ T helper (TH) cells. High IgE production in patients with AE is explained by an impaired balance of the T-cell populations TH1 and TH2, with a predominance of interleukin-4- and interleukin-13-producing TH2 cells [18-23]. Aeroallergens are able to penetrate the disturbed skin barrier[24] in patients with AE and were found in direct contact with antigen-presenting Langerhans cells [25]. The discovery of IgE and IgE-binding structures on the surface of epidermal Langerhans cells[26-29] resulted in a new concept that allergy contributes to the pathophysiology of AE because all of the major components of an IgE-mediated reaction are present in the epidermis. Subsequently, the function of IgE in antigen presentation was shown by Maurer et al.[30] However, the question whether allergy plays a role in practice still remained: measurement of specific serum IgE and skin prick tests or intracutaneous injections of allergen solutions are clinical routine to diagnose IgE-mediated sensitizations,[6,31] but in AE, they reveal often multiple sensitizations without clinical relevance. Furthermore, the morphology of skin test reactions (wheal and flare) does not resemble the clinical manifestation of AE, nor do they represent the appropriate dimensions of the skin immune system. An additional diagnostic tool for aeroallergen-triggered AE was needed, and the proof of concept study was done with a procedure our group called atopy patch test (APT) [32].
Rostenberg and Sulzberger[33] described in 1937 a series of 12,000 patch tests with a wide variety of allergens, including aeroallergens in different patient groups. In 1982, Mitchell et al[34] published the first experimental patch test with aeroallergens for patients with AE. By others, eczematous reactions could be elicited with different methods, but the methodology and definition of positive reactions in these trials were not comparable [35-45]. No clear correlation with history was obtained in larger groups of patients. Potentially irritating procedures like stratum corneum abrasion,[34,46,47] tape stripping,[48-50] or addition of sodium laurylsulfate[51] were necessary to enhance allergen penetration. In 1989, the term atopy patch test was proposed with the following definition: an epicutaneous patch test with allergens known to elicit IgE-mediated reactions, and the evaluation of eczematous skin reactions after 48 and 72 hours [32,52]. The first effort was to standardize APT and possibly develop a method for clinical routine giving positive results only in patients with AE and showing significant concordance to clinical relevance parameters of aeroallergen allergy.
Methodological studies
Allergen lyophilisates of house dust mite Dermatophagoides pteronyssinus, cat dander, grass pollen, and in later studies, of birch and mugwort pollen were used. The test preparations were developed on a noncommercial basis in cooperation with several industrial allergen suppliers to maintain a high standard of batch stability and reproducibility (Hermal and Allergopharma, Reinbek, Germany; Stallergénes, Antony, France). Application in large aluminum Finn chambers (12-mm diameter) on clinically uninvolved, nonabraded, and untreated back skin was superior to the use of small Finn chambers. Reproducibility of elicited APT reactions within a mean of 16 months was 94%. Vehicles were tested in control areas in all patients and remained in general negative. At the beginning, grading of positive APT reactions was done after 48 and 72 hours according to the International Contact Dermatitis Reseach Group rules [53,54]. Only reactions with infiltration were regarded as clear-cut positive (example in Figure 1). All APT studies were performed after discontinuance of systemic antihistamines (the effect of antihistamines on APT is not known to date) and systemic and topical (test area) steroids for at least 7 days.
Figure 1.
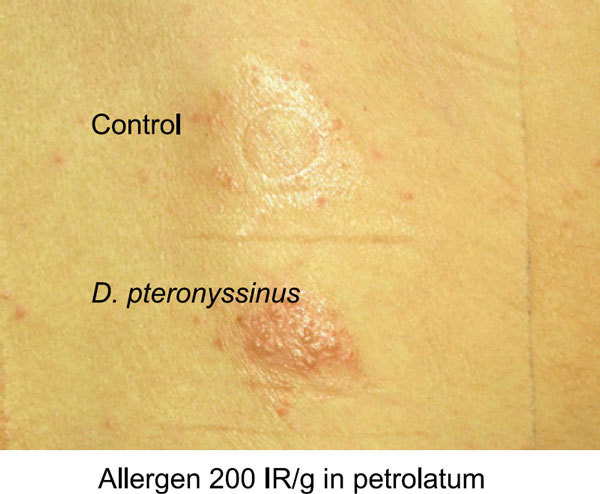
The APT with house dust mite D. pteronyssinus in a patient with AE. Eczematous reaction after 48 hours.
Role of the vehicle
In a pilot study[55] involving 36 patients with AE, the reactions of 17 patients (47%) were graded as clear-cut positive. Control sites (petrolatum, hydrogel) remained negative, non-atopic volunteers and patients with respiratory atopy (allergic rhinoconjunctivitis) only were also negative in APT. Allergens in petrolatum vehicle elicited twice as many positive APT reactions as the same dose in a hydrogel. Thirty-six percent of patients reacted to house dust mite D. pteronyssinus, 22% to cat dander, and 16% to grass pollen. A D. pteronyssinus-positive APT was accompanied in 77% by a corresponding elevated specific IgE (skin prick test, 62%).
Dose-response effects and role of localization of eczema
Allergen concentrations of 500, 3000, 5000, and 10,000 protein nitrogen units (PNU)/g in petrolatum were compared in another study[56] in 57 patients. The frequency of clear-cut positive APT reactions was significantly higher in patients with eczematous skin lesions in air-exposed areas (69%) as compared with patients without this predictive pattern (39%; P = 0.02). In the first group, the maximum APT reactivity was reached at a lower allergen dose of around 5000 PNU/g.
Two hundred fifty-three adult patients with AE (Table 1) participated in a randomized, double-blind, multicenter study on dose-response, safety, and clinical covariates of the APT [57]. The allergen dose with the most clear-cut results (positive or negative) in adults was found for D. pteronyssinus, cat dander, and grass pollen between 5000 and 7000 PNU/g. Most patients reacted only to 1 allergen, rarely to 2 or 3.
Table 1.
Clinical Covariates of the APT in 2 Multicenter Studies With Different Allergen Standardization
| Skin Prick | sIgE | APT | History | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | |
| D. pteronyssinus | 59 | 56 | 56 | 56 | 34 | 39 | 52 | 34 |
| Cat dander | 54 | 44 | 49 | 46 | 12 | 10 | 23 | 30 |
| Grass pollen | 65 | 57 | 75 | 59 | 18 | 15 | 33 | 31 |
| Birch pollen | 65 | 49 | 65 | 53 | 11 | 17 | 13 | 20 |
Age
In 30 children and adolescents 14 years old or younger with AE enrolled in a double-blind dose-response multicenter study,[58] a lower frequency of positive APT reactions compared with adults was seen for D. pteronyssinus (34% vs 41% in adults) and cat dander (12% vs 17%). For D. pteronyssinus and grass pollen, lower allergen doses for APT seem possible in children because maximal response rates were obtained for these allergens with 3000 PNU/g, half of the adult allergen concentration.
Allergen standardization
Comparing different allergen standardization systems in 50 patients with parallel testing, the allergen doses of 7000 PNU/g and 200 IR/g (biological unit) were found to have similar concordance with the patients' clinical history: 71% to 73% of APTwere corroborated by a corresponding positive or negative history of AE flares after contact with the specific allergen. Expressed as major allergen content, 200 IR/g correspond to 59 μg/mL Der p1, 9 μg/mL Fel d1, or 2 μg/mL Phl p1.
In summary, these studies showed that:
• a safe standardized APT method with positive reactions only in patients with AE was developed;
• allergen lyophilisate in petrolatum is the preferred galenic preparation;
• APT is possible on nonabraded skin without manipulation of the skin barrier function;
• allergen concentrations higher than in most prick test solutions are necessary for APT, but lower doses can be used in children;
• D. pteronyssinus is the most frequent allergen eliciting positive APT reactions, with reactions to pollen allergens also being very frequent; and
• high allergen-specific IgE in serum is not a prerequisite for a positive APT.
APT and specific IgE
The percentages of positive reactions in different test systems for IgE-mediated hypersensitivity obtained from our multicenter studies are given in Table 1. These and previous APT studies showed that positive APT occurred less frequently than positive skin prick tests or radioallergosorbent tests (RASTs) to the same allergen. Logistic regression analysis[57] revealed patient's history, skin prick test, and specific corresponding IgE for D. pteronyssinus, cat dander, and grass pollen as most important significant predictors of a positive APT (P < 0.001). However, the cross-tabulation also confirmed that high allergen-specific IgE in serum is not mandatory for a positive APT (example in Table 2), the same holds true for the correlation with skin prick tests. A European multicenter study[59] on standardized APT in 6 countries (n = 314) showed a subgroup of 7% APT-positive AE patients without any positive skin prick test or elevated specific IgE in the investigated allergen panel (Figure 2). Nevertheless, these reactions can be of clinical relevance and immunological specificity [60]. In conclusion,
Table 2.
Cross-Tabulation of APT and Specific IgE to House Dust Mite D. pteronyssinus, Results of a Multicenter Study[57]
| APT 48-h Results in Adult Patients for House Dust Mite D. pteronyssinus | sIgE Negative | sIgE Positive | Total |
|---|---|---|---|
| APT negative | 49 | 29 | 78 |
| APT positive | 13 | 60 | 73 |
| Total | 62 | 89 | 151 |
Figure 2.
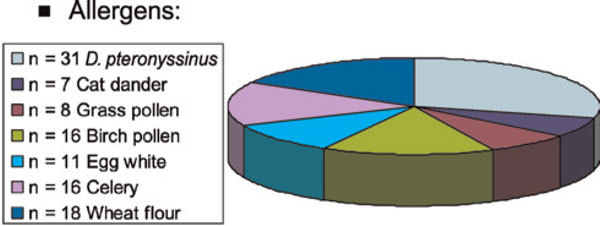
In 53 (17%) of 314 patients of a European multicenter study,[59]positive APT reactions, but negative corresponding skin prick test/specific IgE results, were observed (1 allergen, n = 26; 2 allergens, n = 12). In 22 of these patients with a clear-cut positive APT result, no positive skin prick test or elevated specific serum IgE of the investigated allergen panel was seen (7% of total). The figure shows that all allergens contribute to these reactions.
• the APT may give further diagnostic information in addition to patient's history and classical tests of IgE-mediated hypersensitivity;
• the role for IgE in the reaction mechanism of APT is corroborated because in most APT-positive patients, elevated specific IgE was found compared with those with negative APT; and
• a cellular mechanism without direct involvement of IgE may be hypothesized to explain the clear-cut positive APT reactions in a subgroup of AE patients.
IgE-mediated sensitization and APT: diagnostic precision
Unlike in food allergic patients, a "golden standard" of provocation of aeroallergen-induced AE is not established. The prospectively obtained history of allergen-induced exacerbations of AE, especially in a seasonal allergen, can be used to evaluate the clinical relevance of an APT result like in conventional patch testing. Rajka[3] reported on the phenomenon of "summer eruption," that is, eczema flares during spring and summer, the pollen seasons of birch and grass, in one third of patients with AE. According to our results, one third of patients with specific IgE to grass pollen can have a positive APT reaction to this allergen [57]. In a study on the influence of grass pollen on AE,[61] we tested 79 patients with an APT with 10,000 PNU/g grass pollen allergen mixture in petrolatum and simultaneously with 10 mg of dry unprocessed grass pollen of Dactylis glomerata. Significantly higher frequencies of positive APT occurred in patients with a history of exacerbation of AE in the summer months of the previous year or in direct contact with grass (n = 12, 75% had positive APT) compared with patients without this history (n = 67, 16% had positive APT; P < 0.001). Sensitivity and specificity of APT and classical tests for IgE-mediated sensitization are given in Table 3. The standardized APT also correlated with a predictive eczema pattern, skin prick test, and specific IgE to grass pollen (P < 0.01). Moreover, unprocessed grass pollen also elicited eczematous skin reactions on nonpretreated skin of patients with AE, significantly associated to history and a positive standardized APT with lyophilisate. Again, in healthy and rhinoconjunctivitis controls, no positive reactions were observed.
Table 3.
Sensitivity and Specificity of Different Diagnostic Methods in 2 Studies With Patients With AE
| Test | Sensitivity* | Specificity* |
|---|---|---|
| Single-center study, n = 79 (allergen, grass pollen) | ||
| Skin prick | 100 | 33 |
| RAST | 92 | 33 |
| APT | 75 | 84 |
| APT multicenter study, n = 253 (3 allergens) | ||
| Skin prick | 69-82 | 44-53 |
| RAST | 65-94 | 42-64 |
| APT | 42-56 | 69-92 |
In a larger patient group in the German multicenter study,[57] APT results of D. pteronyssinus, cat dander, and grass pollen were also statistically significantly associated with clinical history (P < 0.001, χ2 and logistic regression; birch pollen, P = 0.1). Thus, sensitivity and specificity of different diagnostic tests could be compared. Allergen-dependent, the APT showed a higher specificity with regard to clinical relevance of an allergen than skin prick test and specific IgE, but also in most allergens, a lower sensitivity (Table 3). In a subgroup of these patients, specific activation and proliferation of T cells in peripheral blood was compared with the patient's APT result [62]. Positive APT reactions were significantly more frequent in patients with elevated CD54+ or CD30+ T cells after in vitro stimulation with the corresponding allergen. In addition, positive APT results were associated with an allergen-specific lymphocyte proliferation (P < 0.001). Positive APT reactions were not associated with disease severity in the SCORAD (scoring atopic dermatitis) system [62,63].
From APT biopsies, allergen-specific T cells have been cloned. In serial biopsies, T cells showed a characteristic TH2 secretion pattern (interleukin-4, -13) at 24 hours, whereas after 48 hours, a TH1 pattern (interferon-γ) like in chronic AE lesions was predominant [64-66]. Taken together, these findings:
• argue against the interpretation of APT results as irritative or nonspecific;
• suggest that pollen are involved in AE flares in some patients previously diagnosed as having UV-triggered eczema;
• demonstrate the clinical relevance of positive APT reactions and the different compartments of allergic inflammation that can be investigated with skin prick test, specific serum IgE determination, and APT;
• show that allergen-specific T cells and IgE play a role in the pathophysiology of APT reactions; and
• sustain the concept that AE is not only a disease of dry skin or barrier dysfunction, but also an allergic disease.
The future of the APT
Appropriate allergen-specific avoidance strategies[13,14,16,67,68] are recommended in patients showing positive APT reactions. The identified subgroup of patients may profit extraordinarily from allergen avoidance, but controlled studies using specific provocation and elimination procedures in patients with positive and negative APT results are still necessary. Standardization of major allergen content and achieving an increase in the sensitivity of APT are important goals of ongoing trials. The combination of APT and specific IgE results may lead to higher diagnostic precision, but the problem of discordant tests has to be solved. Meetings of most European groups performing APT for clinical use in 1997, 1998, and 2003 resulted in a consensus APT reading key of the European Task Force on Atopic Dermatitis (Figure 3) [60,69]. The European multicenter trial also started the investigation of food allergen patch testing with lyophilisates in petrolatum. The development of a standardized APT for food allergy is a future plan, as well as a study on the mechanisms of APT reactions involving both patients with intrinsic and extrinsic AE and investigating the role of the Fcε receptor I and allergen-specific T-cell populations. Furthermore, the APT will be used in trials on topical and systemic therapy for AE and is evaluated as itch model [70,71]. Other aeroallergens of regional significance are to be standardized. A test for the clinical relevance of an aeroallergen sensitization that can be applied in the allergist's practice may evolve soon. The APT may also be valuable in selecting patients for specific immunotherapy. To solve the remaining tasks in due time and to overcome the methodological inconsistencies of different groups, the organization of international multicenter studies is necessary. A recent European Academy of Allergology and Clinical Immunology/Global Allergy and Asthma European Network position paper focused on clinical results and unresolved problems of APT [72].
Figure 3.
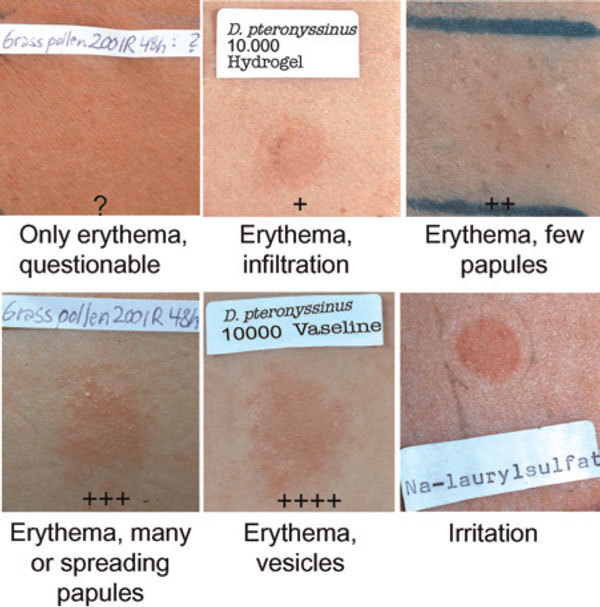
The APT reaction grading key (2003 European Task Force on Atopic Dermatitis consensus).
Concluding remarks
The described APT methodology was evaluated in several hundreds of patients with AE. In a large subgroup of them, IgE-dependent allergic reactions that are elicited by the transdermal route play a pathophysiological role. For patients with aeroallergen-triggered disease, the APT may provide an important diagnostic tool, a provocation test of the skin in analogy to the specific provocation methods in respiratory atopy. As in respiratory atopy, the results of our studies sustain B. Wüthrich's concept of extrinsic/allergic versus intrinsic/idiopathic AE [73].
Positive APT results were obtained in some AE patients with negative skin prick tests and RAST, but predictive history. According to the previously mentioned concept, these cases may also be classified as "extrinsic," and with the back-ground of the recently proposed novel nomenclature for allergy,[74] we suggested to diagnose these cases as "non-IgE-associated AE (dermatitis syndrome)."[60]
Acknowledgements
This work was only possible with the significant contributions of the collaborators and coauthors of the cited studies.
References
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;1:146–148. [Google Scholar]
- Jones HE, Inouye JC, McGerity JL, Lewis CW. Atopic disease and serum immunoglobulin-E. Br J Dermatol. 1975;1:17–25. doi: 10.1111/j.1365-2133.1975.tb03028.x. [DOI] [PubMed] [Google Scholar]
- Rajka G. Essential Aspects of Atopic Dermatitis. Berlin: Springer; 1989. [Google Scholar]
- Hanifin JM. Clinical and basic aspects of atopic dermatitis. Semin Dermatol. 1983;1:5. [Google Scholar]
- Leung DYM, Rhodes AR, Geha RS, Schneider LC, Ring J. In: Dermatology in General Medicine. 4. Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editor. New York: McGraw Hill; 1993. Atopic dermatitis (atopic eczema) pp. 1543–1563. [Google Scholar]
- Ring J. Angewandte Allergologie, 3. Aufl. München: MMV Medizin Verlag; 2004. [Google Scholar]
- Ring J. In: Handbook of Atopic Eczema. 2. Ruzicka T, Ring J, Przybilla B, editor. Berlin, Germany: Springer; 2006. Atopy: condition, disease, or syndrome? pp. 3–9. [Google Scholar]
- Morren MA, Przybilla B, Bamelis M. et al. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;1:467–473. doi: 10.1016/S0190-9622(94)70213-6. [DOI] [PubMed] [Google Scholar]
- Przybilla B, Ring J. Food allergy and atopic eczema. Semin Dermatol. 1990;1:220–225. [PubMed] [Google Scholar]
- Skov L, Baadsgaard O. Ultraviolet B-exposed major histocompatibility complex class II positive keratinocytes and antigen-presenting cells demonstrate a differential capacity to activate T cells in the presence of staphylococcal superantigens. Br J Dermatol. 1996;1:824–830. doi: 10.1111/j.1365-2133.1996.tb06310.x. [DOI] [PubMed] [Google Scholar]
- van Bever HP, Docx M, Stevens WJ. Food and food additives in severe atopic dermatitis. Allergy. 1989;1:588–594. doi: 10.1111/j.1398-9995.1989.tb04205.x. [DOI] [PubMed] [Google Scholar]
- Barnetson RSTC, MacFarlane HAF, Benton EC. House dust mite allergy and atopic eczema: a case report. Br J Dermatol. 1987;1:857–860. doi: 10.1111/j.1365-2133.1987.tb04905.x. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Imayama S, Okada T. Mite-free room (MFR) for the management of atopic dermatitis. Jpn J Allergol. 1991;1:626–632. [PubMed] [Google Scholar]
- Ring J, Brockow K, Abeck D. The therapeutic concept of "patient management" in atopic eczema. Allergy. 1996;1:206–215. [PubMed] [Google Scholar]
- Sanda T, Yasue T, Oohashi M, Yasue A. Effectiveness of house dust-mite allergen avoidance with atopic dermatitis. J Allergy Clin Immunol. 1992;1:653–657. doi: 10.1016/0091-6749(92)90370-H. [DOI] [PubMed] [Google Scholar]
- Tan B, Weald D, Strickland I, Friedman P. Double-blind controlled trial of effect of housedust-mite allergen avoidance on atopic dermatitis. Lancet. 1996;1:15–18. doi: 10.1016/S0140-6736(96)91556-1. [DOI] [PubMed] [Google Scholar]
- Tupker R, DeMonchy J, Coenraads P, Homan A, van der Meer J. Induction of atopic dermatitis by inhalation of house dust mite. J Allergy Clin Immunol. 1996;1:1064–1070. doi: 10.1016/S0091-6749(96)70259-2. [DOI] [PubMed] [Google Scholar]
- Grewe M, Gyufko K, Schöpf E, Krutmann J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994;1:25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- Leung DYM, Ghan AK, Schneeberger EE, Geha RS. Characterization of the mononuclear cell infiltrate in atopic dermatitis using mononuclear antibodies. J Allergy Clin Immunol. 1983;1:47–56. doi: 10.1016/0091-6749(83)90546-8. [DOI] [PubMed] [Google Scholar]
- Ohmen JD, Hanifin JM, Nickoloff BJ. et al. Overexpression of IL-10 in atopic dermatitis. Contrasting cytokine patterns with delayed-type hypersensitivity reactions. J Immunol. 1995;1:1956–1963. [PubMed] [Google Scholar]
- Renz H, Jujo K, Bradley K. et al. Enhanced IL-4 production and IL-4 receptor expression in atopic dermatitis and their modulation by interferon-gamma. J Invest Dermatol. 1992;1:403–408. doi: 10.1111/1523-1747.ep12616114. [DOI] [PubMed] [Google Scholar]
- Sowden J, Powell R, Allen B. Selective activation of circulating CD4+ lymphocytes in severe adult atopic dermatitis. Br J Dermatol. 1992;1:228–232. doi: 10.1111/j.1365-2133.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Vercelli D, Jabara H, Lauener R, Geha R. IL-4 inhibits the synthesis of INF-gamma and induces the synthesis of IgE in human mixed lymphocyte cultures. J Immunol. 1990;1:570–573. [PubMed] [Google Scholar]
- Schäfer L, Kragballe K. Abnormalities in epidermal lipid metabolism in patients with atopic dermatitis. J Invest Dermatol. 1991;1:10–15. doi: 10.1111/1523-1747.ep12514648. [DOI] [PubMed] [Google Scholar]
- Maeda K, Yamamoto K, Tanaka Y, Anan S, Yoshida H. House dust mite (HDM) antigen in naturally occurring lesions of atopic dermatitis (AD): the relationship between HDM antigen in the skin and HDM antigen-specific IgE antibody. J Dermatol Sci. 1992;1:73–77. doi: 10.1016/0923-1811(92)90038-D. [DOI] [PubMed] [Google Scholar]
- Bieber T, de la Salle C, Wollenberg A, Hakimi J, Chizzonite R, Ring J. Constitutive expression of the high affinity receptor for IgE (FCeR1) on human Langerhans-cells. J Exp Med. 1992;1:1285–1290. doi: 10.1084/jem.175.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber T, Rieger A, Neuchrist C. et al. Induction of FCeR2/CD23 on human epidermal Langerhans cells by human recombinant IL4 and IFN. J Exp Med. 1989;1:309–314. doi: 10.1084/jem.170.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber T. FCeRI on human Langerhans cells: a receptor in seach of new functions. Immunol Today. 1994;1:52–53. doi: 10.1016/0167-5699(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel-Koomen C, van Wichen DF, Toonstra J, Berrens L, Bruijnzeel PLB. The presence of IgE molecules on epidermal Langerhans cells in patients with atopic dermatitis. Arch Dermatol Res. 1986;1:199–205. doi: 10.1007/BF00412924. [DOI] [PubMed] [Google Scholar]
- Maurer D, Ebner C, Reininger B, Fiebiger E, Kraft D, Kinet Jp, Stingl G. The high affinity IgE receptor mediates IgE-dependent allergen presentation. J Immunol. 1995;1:6285–6290. [PubMed] [Google Scholar]
- Darsow U. In: Neurodermitis. Ring J, editor. Landsberg: Ecomed; 1998. Etablierte Diagnostikverfahren; pp. 61–73. [Google Scholar]
- Ring J, Kunz B, Bieber T, Vieluf D, Przybilla B. The "atopy patch test" with aeroallergens in atopic eczema. J Allergy Clin Immunol. 1989;1:195. [abstract] [Google Scholar]
- Rostenberg A, Sulzberger MD. Some results of patch tests. Arch Dermatol. 1937;1:433–454. doi: 10.1001/archderm.1937.01470210059006. [DOI] [Google Scholar]
- Mitchell E, Chapman M, Pope F, Crow J, Jouhal S, Platts-Mills T. Basophils in allergen-induced patch test sites in atopic dermatitis. Lancet. 1982;1:127–130. doi: 10.1016/s0140-6736(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Adinoff A, Tellez P, Clark R. Atopic dermatitis and aeroallergen contact sensitivity. J Allergy Clin Immunol. 1988;1:736–742. doi: 10.1016/0091-6749(88)91047-0. [DOI] [PubMed] [Google Scholar]
- Clark R, Adinoff A. Aeroallergen contact can exacerbate atopic dermatitis: patch test as a diagnostic tool. J Am Acad Dermatol. 1989;1:863–869. doi: 10.1016/S0190-9622(89)70269-3. [DOI] [PubMed] [Google Scholar]
- Imayama S, Hashizuma T, Miyahara H. et al. Combination of patch test and IgE for dust mite antigens differentiates 130 patients with atopic dermatitis into four groups. J Am Acad Dermatol. 1992;1:531–538. doi: 10.1016/0190-9622(92)70218-5. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T, Mitchell E, Rowntree S, Chapman M, Wilkins S. The role of dust mite allergens in atopic dermatitis. Clin Exp Dermatol. 1983;1:233–247. doi: 10.1111/j.1365-2230.1983.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Reitamo S, Visa K, Kaehoenen K, Käykhö A, Lauerna I, Stubb S, Salo OP. Patch test reactions to inhalant allergens in atopic dermatitis. Acta Derm Venereol. 1989;1:119–121. [PubMed] [Google Scholar]
- Reitamo S, Visa K, Kähönen K, Stubb S, Salo OP. Eczematous reactions in atopic patients caused by epicutaneous testing with inhalant allergens. Br J Dermatol. 1986;1:303–309. doi: 10.1111/j.1365-2133.1986.tb02821.x. [DOI] [PubMed] [Google Scholar]
- Seidenari S, Manzini BM, Danese P, Giannetti A. Positive patch tests to whole mite culture and purified mite extracts in patients with atopic dermatitis, asthma and rhinitis. Ann Allergy. 1992;1:201–206. [PubMed] [Google Scholar]
- Seidenari S, Manzini M, Danese P. Patch testing with pollens of Gramineae in patients with atopic dermatitis and mucosal atopy. Contact Dermatitis. 1992;1:125–126. doi: 10.1111/j.1600-0536.1992.tb05232.x. [DOI] [PubMed] [Google Scholar]
- Seifert H, Wollemann G, Seifert B, Borelli S. Neurodermitis: Eine Protein-Kontaktdermatitis? Dtsch Derm. 1987;1:1204–1214. [Google Scholar]
- Vieluf D, Kunz B, Bieber T, Przybilla B, Ring J. Atopy patch test with aeroallergens in patients with atopic eczema. Allergo J. 1993;1:9–12. [Google Scholar]
- Vocks E, Seifert H, Seifert B, Drosner M. In: New Trends in Allergy III. Ring J, Przybilla B, editor. Berlin: Springer; 1991. Patch test with immediate type allergens in patients with atopic dermatitis; pp. 230–233. [Google Scholar]
- Gondo A, Saeki N, Tokuda Y. Challenge reactions in atopic dermatitis after percutaneous entry of mite antigen. Br J Dermatol. 1986;1:485–493. doi: 10.1111/j.1365-2133.1986.tb06243.x. [DOI] [PubMed] [Google Scholar]
- Norris P, Schofield O, Camp R. A study of the role of house dust mite in atopic dermatitis. Br J Dermatol. 1988;1:435–440. doi: 10.1111/j.1365-2133.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel-Koomen C, van Wichen D, Spry C, Venge P, Bruynzeel P. Active participation of eosinophils in patch test reactions to inhalant allergens in patients with atopic dermatitis. Br J Dermatol. 1988;1:229–238. doi: 10.1111/j.1365-2133.1988.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Langeland T, Braathen L, Borch M. Studies of atopic patch tests. Acta Derm Venereol. 1989;1:105–109. doi: 10.2340/00015555144105109. [DOI] [PubMed] [Google Scholar]
- van Voorst Vader PC, Lier JG, Woest TE, Coenraads PJ, Nater JP. Patch tests with house dust mite antigens in atopic dermatitis patients: methodological problems. Acta Derm Venereol. 1991;1:301–305. [PubMed] [Google Scholar]
- Tanaka Y, Anan S, Yoshida H. Immunohistochemical studies in mite antigen-induced patch test sites in atopic dermatitis. J Dermatol Sci. 1990;1:361–368. doi: 10.1016/0923-1811(90)90593-3. [DOI] [PubMed] [Google Scholar]
- Ring J, Bieber T, Vieluf D, Kunz B, Przybilla B. Atopic eczema, Langerhans cells and allergy. Int Arch Allergy Appl Immunol. 1991;1:194–201. doi: 10.1159/000235361. [DOI] [PubMed] [Google Scholar]
- Andersen KE. et al. The European Environmental and Contact Dermatitis Research Group. Contact dermatitis: a review. Contact Dermatitis. 1987;1:55–78. doi: 10.1111/j.1600-0536.1987.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Fisher AA. Contact Dermatitis. 3. Philadelphia: Lea & Febiger; 1986. pp. 686–691. [Google Scholar]
- Darsow U, Vieluf D, Ring J. Atopy patch test with different vehicles and allergen concentrations--an approach to standardization. J Allergy Clin Immunol. 1995;1:677–684. doi: 10.1016/S0091-6749(95)70172-9. [DOI] [PubMed] [Google Scholar]
- Darsow U, Vieluf D, Ring J. The atopy patch test: an increased rate of reactivity in patients who have an air-exposed pattern of atopic eczema. Br J Dermatol. 1996;1:182–186. doi: 10.1111/j.1365-2133.1996.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Darsow U, Vieluf D, Ring J. for the APT study group. Evaluating the relevance of aeroallergen sensitization in atopic eczema using the tool "atopy patch test": a randomized, double-blind multicenter study. J Am Acad Dermatol. 1999;1:187–193. doi: 10.1016/S0190-9622(99)70186-6. [DOI] [PubMed] [Google Scholar]
- Darsow U, Vieluf D, Berg B. et al. Dose response study of atopy patch test in children with atopic eczema. Pediatr Asthma Allergy Immunol. 1999;1:115–122. doi: 10.1089/pai.1999.13.115. [DOI] [Google Scholar]
- Darsow U, Laifaoui J, Bolhaar S. et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy. 2004;1:1318–1325. doi: 10.1111/j.1398-9995.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Kerschenlohr K, Günther S, Darsow U, Ollert M, Wollenberg A. Clinical and immunological reactivity to aeroallergens in "intrinsic" atopic dermatitis patients. J Allergy Clin Immunol. 2003;1:195–197. doi: 10.1016/S0091-6749(03)70068-2. [DOI] [PubMed] [Google Scholar]
- Darsow U, Behrendt H, Ring J. Gramineae pollen as trigger factors of atopic eczema: evaluation of diagnostic measures using the atopy patch test. Br J Dermatol. 1997;1:201–207. doi: 10.1046/j.1365-2133.1997.18061889.x. [DOI] [PubMed] [Google Scholar]
- Wistokat-Wülfing A, Schmidt P, Darsow U, Ring J, Kapp A, Werfel T. Atopy patch test reactions are associated with T lymphocyte-mediated allergen-specific immune responses in atopic dermatitis. Clin Exp Allergy. 1999;1:513–521. doi: 10.1046/j.1365-2222.1999.00510.x. [DOI] [PubMed] [Google Scholar]
- European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Dermatology. 1993;1:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- Ramb-Lindhauer CH, Feldmann A, Rotte M, Neumann CH. Characterization of grass pollen reactive T-cell lines derived from lesional atopic skin. Arch Dermatol Res. 1991;1:71–76. doi: 10.1007/BF00371611. [DOI] [PubMed] [Google Scholar]
- Sager N, Feldmann A, Schilling G, Kreitsch P, Neumann C. House dust mite-specific T cells in the skin of subjects with atopic dermatitis: frequency and lymphokine profile in the allergen patch test. J Allergy Clin Immunol. 1992;1:801–810. doi: 10.1016/0091-6749(92)90434-4. [DOI] [PubMed] [Google Scholar]
- van Reijsen FC, Bruynzeel-Koomen CAFM, Kalthoff FS, Maggi E, Romagnani S, Westland JKT, Mudde GC. Skin-derived aeroallergen-specific T-cell clones of TH2 phenotype in patiens with atopic dermatitis. J Allergy Clin Immunol. 1992;1:184–192. doi: 10.1016/0091-6749(92)90070-I. [DOI] [PubMed] [Google Scholar]
- Weissenbacher S, Bacon T, Targett D, Behrendt H, Ring J, Darsow U. Atopy patch test--reproducibility and elicitation of itch in different application sites. Acta Derm Venereol. 2005;1:147–151. doi: 10.1080/00015550410024418. [DOI] [PubMed] [Google Scholar]
- Lau S, Ehnert B, Cremer B, Nasert S, Büttner P, Czarnetzki BM, Wahn U. Häusliche Milbenallergenreduktion bei spezifisch sensibilisierten Patienten mit atopischem Ekzem. Allergo J. 1995;1:432–435. [Google Scholar]
- Darsow U, Ring J. Airborne and dietary allergens in atopic eczema: a comprehensive review of diagnostic tests. Clin Exp Dermatol. 2000;1:544–551. doi: 10.1046/j.1365-2230.2000.00695.x. [DOI] [PubMed] [Google Scholar]
- Weissenbacher S, Bacon T, Targett D, Behrendt H, Ring J, Darsow U. Atopy patch test--reproducibility and elicitation of itch in different application sites. Acta Derm Venereol. 2005;1:147–151. doi: 10.1080/00015550410024418. [DOI] [PubMed] [Google Scholar]
- Weissenbacher S, Traidl-Hoffmann C, Eyerich K. et al. Modulation of atopy patch test and skin prick test by pretreatment with 1% pimecrolimus cream. Int Arch Allergy Immunol. 2006;1:239–244. doi: 10.1159/000093249. [DOI] [PubMed] [Google Scholar]
- Turjanmaa K, Darsow U, Niggemann B, Rancé F, Vanto T, Werfel T. EAACI/GA2LEN Position Paper: present status of the atopy patch test--position paper of the Section on Dermatology and the Section on Pediatrics of the EAACI. Allergy. 2006;1:1377–1384. doi: 10.1111/j.1398-9995.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Wüthrich B. Neurodermitis atopica sive constitutionalis. Ein pathogenetisches Modell aus der Sicht des Allergologen. Akt Dermatol. 1983;1:1–7. [Google Scholar]
- Johansson SGO, Bieber T, Dahl R. et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;1:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]


