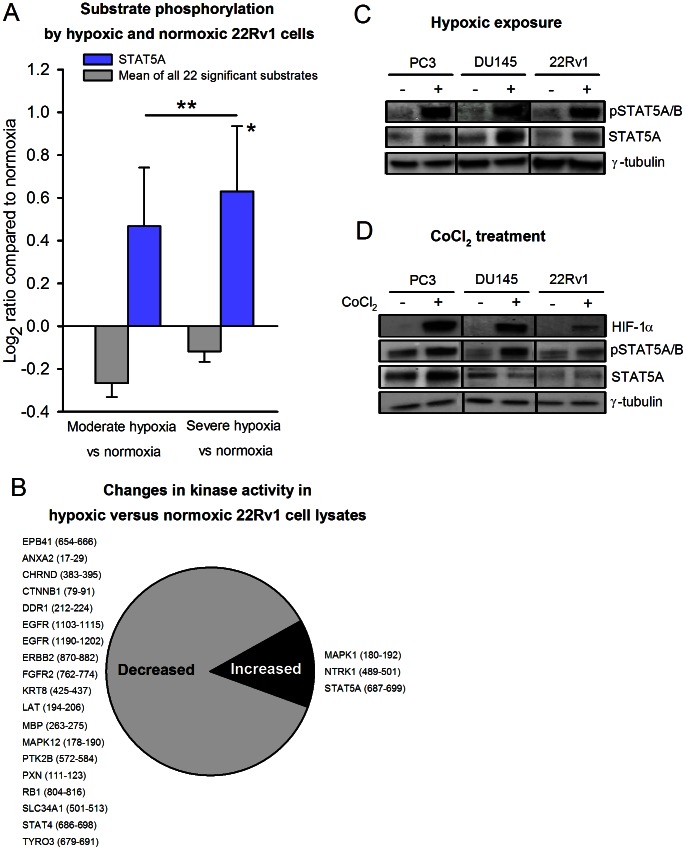
Figure 5. Hypoxia-induced increase in STAT5A substrate phosphorylation by prostate carcinoma cells.
(A) 22Rv1 cells were exposed to moderate (1% O2) and severe (<0.02% O2) hypoxia for 24 hours in a hypoxia chamber. Protein lysates from three biological replicates from each of the three experimental groups (normoxia, moderate hypoxia, severe hypoxia) were analyzed to generate the kinase activity profiles. Kinase activity profiling of hypoxic and normoxic cell lysates detected increased STAT5A substrate phosphorylation with increasing hypoxia (blue bars), whereas the mean phosphorylation level for all 22 kinase peptide substrates with significant changes was low (grey bars). *The increase from normoxia to severe hypoxia was significant (p = 0.020). The difference in mean phosphorylation level at moderate versus severe hypoxia was not significant. (B) The proportion of decreased versus increased kinase activity in hypoxic versus normoxic 22Rv1 cell lysates. Listed are the gene names of the affected kinase peptide substrates, with start and end positions for the peptide sequence within the protein in brackets. (C) Western blot of anti-phospho-STAT5A/B and anti-STAT5A expression in normoxic (−) and severely hypoxic (<0.02% O2, 24 h) (+) cell lysates from PC3, DU145 and 22Rv1 cells. Anti-γ-tubulin served as loading control. (D) Western blot of anti-HIF-1α, anti-phospho-STAT5A/B and anti-STAT5A expression of lysates from PC3, DU145 and 22Rv1 cells with (+) or without (−) 4 hours of exposure to 100 µM of the hypoxia-mimetic agent cobalt chloride (CoCl2), a chemical inducer of HIF-1α. Anti-γ-tubulin served as loading control.