Abstract
Background
Indacaterol is a novel, once-daily (od), inhaled, long-acting ß2-agonist bronchodilator for maintenance treatment of airflow limitation in patients with COPD. The aim of this study was to evaluate the efficacy of indacaterol on dyspnea, using available randomized placebo-controlled trials.
Methods
A systematic search was made of MEDLINE, EMBASE, the Cochrane trials databases, and a manual search of journals. Randomized placebo-controlled trials of 12 weeks or more comparing indacaterol with placebo were reviewed, and eligible studies were included in a meta-analysis. The odds ratio (OR) for likelihood of achieving TDI score ≥ 1 after 12 weeks of treatment was used as an outcome measure to compare indacaterol to placebo.
Results
Six trials were included in the analysis. Relative to placebo, the overall ORs for response were: indacaterol 75 μg od 1.784 (95% CI 1.282 to 2.482); indacaterol 150 μg od 2.149 (95% CI 1.746 to 2.645); and indacaterol 300 μg od 2.458 (95% CI 2.010 to 3.006). Overall OR for response in TDI tended to increase with higher indacaterol doses.
Conclusions
Patients receiving indacaterol had clinically significant improvements in symptoms of dyspnea compared to placebo. Incremental benefits in TDI were observed with increasing doses. Indacaterol may provide patients and physicians with a useful treatment option in symptomatic patients with dyspnea.
Keywords: Breathlessness, Baseline dyspnea index, Transition dyspnea index, Meta-analysis, COPD
Background
The mortality, morbidity and economic burden of chronic obstructive pulmonary disease (COPD) is well documented [1,2]. Dyspnea as a cardinal symptom of COPD is a major cause of disability and anxiety associated with the disease, prompting the medical community to pursue effective treatments for the relief of dyspnea [3]. Recent guidelines recommend the regular use of inhaled long-acting bronchodilators to alleviate dyspnea in patients with symptomatic disease, with the addition of inhaled corticosteroids for patients who experience repeated exacerbations [1,2].
Indacaterol as a novel, once-daily (od), inhaled, long-acting ß2-agonist provides sustained bronchodilation for patients with moderate to severe COPD, with a rapid onset following the first dose [4,5]. It has been recently approved in Europe at doses of 150 μg and 300 μg od, in the United States at 75 μg od, and in China at 150 μg od for maintenance treatment of airflow limitation in patients with COPD. A number of randomized clinical trials suggest that indacaterol may improve dyspnea in patients with stable COPD, as indicated by changes in transition dyspnea index (TDI) [5-10]. However, all of these trials were statistically powered on forced expiratory volume in 1 second (FEV1) as a primary endpoint. The outcome measure of TDI was included as one of the secondary endpoints in these trials, and so might not have been adequately powered.
Therefore, we undertook a systematic review and meta-analysis of available randomized placebo-controlled studies to assess the efficacy of indacaterol on the important clinical outcome of dyspnea. The objectives of this meta-analysis were to combine data from existing randomized placebo-controlled trials, to use the number of patients achieving the minimum clinically important difference (MCID) for TDI score ≥ 1 as an outcome measure, and to evaluate the efficacy of once-daily indacaterol of licensed 75 μg, 150 μg, or 300 μg doses relative to placebo on dyspnea in patients with stable COPD.
Methods
Data sources and selection criteria
We identified published studies between January 2007 and May 2012 from MEDLINE, EMBASE, and the Cochrane Controlled Trials Register (CENTRAL) databases using the terms indacaterol, long-acting ß2-agonist AND chronic obstructive pulmonary disease. We performed a search of relevant files from the Novartis trials results database (http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp). We also performed a manual search of references cited in published original and review articles, and in clinical practice guidelines. Trials published solely in abstract form were excluded because they contain preliminary and rudimentary information and may not provide enough details to allow full analysis. Two reviewers (JNH and LD) then independently screened potentially relevant trials from titles and abstracts. Using the full texts as necessary, the two reviewers independently identified eligible articles for full review. Finally, we reviewed eligible articles to determine whether they qualified for meta-analysis. Differences were resolved by discussion.
To be included, studies had to meet all the following criteria: a) target population of stable COPD consistent with American Thoracic Society/European Respiratory Society [11] or Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria [12]; b) randomized placebo-controlled trials comparing indacaterol 75 μg, 150 μg, or 300 μg od with placebo; c) studies that followed patients for 12 weeks or more after randomization; d) studies that included assessment of dyspnea by transition dyspnea index (TDI) in outcome measures.
Data extraction
Two reviewers independently read each article that met inclusion criteria and performed data extraction using a pre-designed data collection form. Missing data were obtained from the manufacturer. Disagreement and uncertainty were solved by discussion. Consensus was reached for all data. Data extracted from each article included: first author’s name and year of publication; study design; treatment arms; number of patients; treatment duration; baseline clinical characteristics including age, gender, smoking history, post-bronchodilator spirometry, and total score of the baseline dyspnea index (BDI). The outcome measure of TDI was recorded as total score and as the number of patients with TDI ≥ 1.
Baseline dyspnea index (BDI) and transition dyspnea index (TDI)
Dyspnea is often measured using the BDI and TDI, a tool recommended by regulatory authorities for inclusion in clinical trials of treatments for COPD [13]. The BDI and TDI, as multidimensional instruments, each has three domains: functional impairment, magnitude of task, and magnitude of effort [14]. The BDI domains measure baseline dyspnea severity, and are rated from 0 (severe) to 4 (unimpaired) and summed to provide a BDI total score of 0 to 12, with a lower score indicating more severe dyspnea. The TDI domains measure change from the baseline dyspnea index (BDI) over time, rated on a scale of +3 (major improvement) to −3 (major deterioration). The TDI has been shown to be valid, reliable and responsive [14,15]. The minimum clinically important difference (MCID) for the TDI is an improvement from the BDI score of ≥1 unit [16,17].
Statistical analyses
Odds ratio (OR) for likelihood of achieving TDI score ≥1 after 12 weeks of treatment was used as a measure to compare indacaterol relative to placebo. We calculated pooled ORs with the DerSimonian-Laird random effects model [18], usually regarded as more appropriate than other statistical approaches when potential heterogeneity is present between studies [19,20]. We performed separate analyses for indacaterol 75 μg versus placebo, indacaterol 150 μg versus placebo, and indacaterol 300 μg versus placebo. We calculated the 95% confidence intervals around the ORs, and assessed heterogeneity across studies with the chi-square test and I2 (p < 0.10, I2 > 25%). We then created forest plots of the individual studies and combined estimates. All analyses were performed with meta-analysis software (MetaAnalyst version beta 3.13, Tufts Medical Center, Boston, Massachusetts).
Results
Characteristics of included studies
Six studies met the inclusion criteria [5-9], the designs of which are summarized in Table 1. The studies varied from 12 to 52 weeks in duration, and so to permit comparison across trials, assessments at 12 weeks of treatment were used for the 52-week study [6] and for two 26-week studies [5,7]. We did not consider data from tiotropium, formoterol, and salmeterol arms for the current meta-analysis, since data for each of these arms would be provided from one study.
Table 1.
Summary of study designs for the studies included in this meta-analysis
| Study | Design | Treatment arms | Number of patients | Treatment duration |
|---|---|---|---|---|
| Donohue [5] |
Phase III, randomized, double-blind (indacaterol and placebo) or open-label (tiotropium), placebo-controlled, parallel-group, multicentre |
Indacaterol 150 μg od |
1683 randomized; |
26 weeks |
| Indacaterol 300 μg od |
1665 evaluable for efficacy |
|||
| Tiotropium 18 μg od |
|
|||
| Placebo | ||||
| Dahl [6] |
Phase III, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicentre |
Indacaterol 300 μg od |
1732 randomized; |
52 weeks |
| Indacaterol 600 μg od |
1600 evaluable for efficacy |
|||
| Formoterol 12 μg bid | ||||
| Placebo | ||||
| Kornmann [7] |
Phase III, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, multicentre |
Indacaterol 150 μg od |
1002 randomized; |
26 weeks |
| Salmeterol 50 μg bid |
998 evaluable for efficacy |
|||
| Placebo | ||||
| Gotfried-1 [8] |
Phase III, randomized, double-blind, placebo-controlled, parallel-group, in the United States |
Indacaterol 75 μg od |
323 randomized; |
12 weeks |
| Placebo |
323 evaluable for efficacy |
|||
| Gotfried-2 [8] |
Phase III, randomized, double-blind, placebo-controlled, parallel-group, in the United States |
Indacaterol 75 μg od |
318 randomized; |
12 weeks |
| Placebo |
317 evaluable for efficacy |
|||
| Kinoshita [9] |
Phase III, randomized, double-blind, placebo-controlled, parallel-group, in six Asian areas |
Indacaterol 150 μg od |
347 randomized; |
12 weeks |
| Indacaterol 300 μg od |
347 evaluable for efficacy |
|||
| Placebo |
od = once-daily; bid = twice-daily.
Table 2 illustrates patient populations and baseline characteristics. Of the six studies providing the data for the meta-analysis, four had similar inclusion criteria [5-7,9], recruiting male and female patients aged ≥40 years with a clinical diagnosis of moderate-to-severe COPD as per the GOLD 2005 criteria and a smoking history of ≥20 pack-years. Postbronchodilator FEV1 was to be <80% and ≥30% predicted and post-bronchodilator FEV1/forced vital capacity <70% [5-7,9]. The two identical indacaterol 75 μg trials enrolled patients with moderate-to-severe COPD defined at that time using GOLD 2008 criteria, aged ≥40 years and with a smoking history of ≥10 pack-years [8].
Table 2.
Patient characteristics at baseline in the individual studies
| Study | Treatment arms | Patients, na | Age, years | Male, n (%) | Smoking history, pack-years | FEV1, Lb | FEV1, % predb | FEV1/FVC, %b | BDI total scorec |
|---|---|---|---|---|---|---|---|---|---|
| Donohue [5] |
Indacaterol 150 μg od |
416 |
63.4 (40, 87) |
259 (62.3) |
48.3 (20, 150) |
1.52 (0.62, 3.45) |
56.1( 29.3, 116.6) |
53.0 (24.4, 69.7) |
6.56 (0, 12) |
| Indacaterol 300 μg od |
416 |
63.3 (40, 88) |
263 (63.2) |
50.8 (13, 208) |
1.53 (0.57, 3.14) |
56.3 (21.3, 90.0) |
52.6 (25.7, 69.5) |
6.52 (0, 12) |
|
| Tiotropium 18 μg od |
415 |
64.0 (41, 85) |
269 (64.8) |
50.0 (20, 180) |
1.45 (0.48, 3.00) |
53.9 (23.6, 132.3) |
52.7 (24.7, 72.6) |
6.57 (0, 12) |
|
| Placebo |
418 |
63.6 (41, 84) |
255 (61.0) |
49.7 (20, 156) |
1.51 (0.53, 2.98) |
56.1 (28.4, 95.1) |
53.4 (24.0, 69.9) |
6.39 (0, 12) |
|
| Dahl [6] |
Indacaterol 300 μg od |
437 |
63.9 (40, 87) |
351 (80.3) |
48.6 (20, 600) |
1.48 (0.44, 2.95) |
52.8 (23.5, 101.4) |
51.1 (27.7, 90.1) |
6.62 (0, 12) |
| Indacaterol 600 μg od |
425 |
62.9 (40, 87) |
327 (76.9) |
53.6 (20, 900) |
1.48 (0.55, 2.91) |
51.6 (24.0, 84.2) |
51.1 (15.8, 84.4) |
6.57 (0, 12) |
|
| Formoterol 12 μg bid |
434 |
63.6 (40, 84) |
348 (80.2) |
49.0 (20, 800) |
1.50 (0.59, 3.25) |
52.9 (20.8, 100.5) |
51.3 (23.0, 96.5) |
6.46 (1, 12) |
|
| Placebo |
432 |
63.2 (41, 90) |
352 (81.5) |
53.3 (20, 900) |
1.52 (0.58, 3.09) |
52.9 (17.6, 96.3) |
52.1 (21.5, 80.0) |
6.52 (0, 12) |
|
| Kornmann [7] |
Indacaterol 150 μg od |
330 |
63.2 (41, 85) |
238 (72.1) |
39.6 (20, 120) |
1.48 (0.63, 2.93) |
53.9 (30.0, 104.4) |
53.5 (23.5, 76.8) |
6.74 (0, 12) |
| Salmeterol 50 μg bid |
333 |
63.4 (41, 86) |
249 (74.8) |
40.0 (20, 147) |
1.48 (0.43, 3.18) |
53.1 (17.9, 93.4) |
52.2 (18.0, 82.2) |
6.68 (0, 12) |
|
| Placebo |
335 |
63.9 (42, 89) |
258 (77.0) |
41.0 (20, 159) |
1.46 (0.47, 3.20) |
53.0 (12.3, 91.0) |
52.7 (20.2, 90.9) |
6.63 (0, 12) |
|
| Gotfried-1 [8] |
Indacaterol 75 μg od |
163 |
64.0 (44, 85) |
89 (54.6) |
52.9 (10, 150) |
1.49 (0.57, 2.92) |
53.7 (30.3, 77.3) |
53.1 (31.8, 68.3) |
6.40 (2, 12) |
| Placebo |
160 |
64.1 (40, 90) |
87 (54.4) |
51.2 (10, 148) |
1.46 (0.62, 2.77) |
53.3 (29.5, 78.7) |
51.6 (25.6, 69.1) |
5.81 (1, 12) |
|
| Gotfried-2 [8] |
Indacaterol 75μg od |
159 |
61.3 (40, 82) |
83 (52.2) |
52.4 (11, 180) |
1.59 (0.65, 3.39) |
55.7 (29.7, 79.3) |
52.4 (22.9, 68.6) |
6.01 (0, 12) |
| Placebo |
158 |
61.5 (42, 86) |
89 (56.0) |
52.4 (10, 204) |
1.52 (0.56, 3.00) |
53.5 (30.3, 79.4) |
52.6 (28.8, 69.1) |
6.15 (1, 11) |
|
| Kinoshita [9] |
Indacaterol 150 μg od |
114 |
66.4 (46, 83) |
110 (96.5) |
51.7 (20, 196) |
1.46 (0.70, 2.50) |
55.2 (30.0, 79.0) |
50.3 (27.0, 69.0) |
7.53 (1, 12) |
| Indacaterol 300 μg od |
116 |
67.1 (48, 86) |
113 (97.4) |
54.0 (20, 150) |
1.41 (0.68, 2.92) |
53.7 (30.0, 79.0) |
48.7 (27.0, 69.0) |
7.67 (1, 12) |
|
| Placebo | 117 | 66.5 (40, 88) | 112 (95.7) | 49.7 (20, 160) | 1.38 (0.55, 2.37) | 52.3 (30.0, 77.0) | 47.7 (28.0, 69.0) | 7.35 (1, 12) |
Data are mean (min, max) for age, smoking history, FEV1, FEV1 % predicted, and FEV1/FVC% unless stated otherwise.
a Number of patients analysed for efficacy (ITT, modified ITT or FAS).
b Post-bronchodilator spirometry.
c Values for patients who provided data at Week 12.
Dyspnea – BDI and TDI
In all trials included in the meta-analysis, dyspnea was measured at baseline using the BDI. As illustrated in Table 2, patients had moderate severity of dyspnea at baseline with mean BDI total scores ranging from 5.81 to 7.67. After 12 weeks of treatment, dyspnea was measured using the TDI (Table 3), which captured changes from baseline. Data are presented as mean TDI total scores and as the number of patients with TDI score ≥1 unit. Results were analyzed for the number of patients responding with a change of TDI equal to or greater than the MCID (‘responder analysis’) (Table 3).
Table 3.
Indacaterol on dyspnea as measured by the TDI
|
Study |
Treatment arms |
TDI at week 12 |
||
|---|---|---|---|---|
| Patients, na | TDI total scoreb | Number of patients with TDI ≥1 | ||
| Donohue [5] |
Indacaterol 150 μg od |
355 |
2.09 (-8, 9) |
209 |
| Indacaterol 300 μg od |
363 |
2.40 (-9, 9) |
239 |
|
| Tiotropium 18 μg od |
360 |
1.89 (-6, 9) |
198 |
|
| Placebo |
326 |
1.19 (-6, 9) |
138 |
|
| Dahl [6] |
Indacaterol 300 μg od |
364 |
2.11 (-9, 9) |
229 |
| Indacaterol 600 μg od |
348 |
2.10 (-7, 9) |
202 |
|
| Formoterol 12 μg bid |
359 |
1.64 (-9, 9) |
190 |
|
| Placebo |
343 |
0.87 (-9, 9) |
138 |
|
| Kornmann [7] |
Indacaterol 150 μg od |
303 |
2.37 (-6, 9) |
182 |
| Salmeterol 50 μg bid |
296 |
1.60 (-6, 9) |
152 |
|
| Placebo |
286 |
0.87 (-9, 9) |
113 |
|
| Gotfried-1 [8] |
Indacaterol 75 μg od |
150 |
1.36 (-6, 9) |
73 |
| Placebo |
150 |
0.12 (-9, 9) |
48 |
|
| Gotfried-2 [8] |
Indacaterol 75 μg od |
148 |
1.25 (-7, 9) |
69 |
| Placebo |
149 |
0.83 (-6, 9) |
53 |
|
| Kinoshita [9] |
Indacaterol 150 μg od |
108 |
2.16 (-3, 9) |
66 |
| Indacaterol 300 μg od |
107 |
2.04 (-3, 9) |
58 |
|
| Placebo | 102 | 0.88 (-6, 9) | 40 | |
a Number of patients evaluated for TDI total score at week 12 and included in the responder analysis.
b Values are mean (min, max) of raw and unadjusted TDI total score.
Indacaterol 75 μg versus placebo
Two randomized placebo-controlled studies had identical entry criteria and study designs, which compared indacaterol 75 μg once daily with placebo after 12 weeks of treatment [8]. A meta-analysis that combined the two studies produced a pooled OR estimate of 1.784 (95% CI 1.282–2.482) with no evidence of heterogeneity (P = 0.474, I2 = 0.000), indicating that relative to placebo, patients receiving indacaterol 75 μg are more likely to achieve TDI score ≥1 after 12 weeks of treatment. Figure 1 shows a forest plot of OR estimates from these studies.
Figure 1.
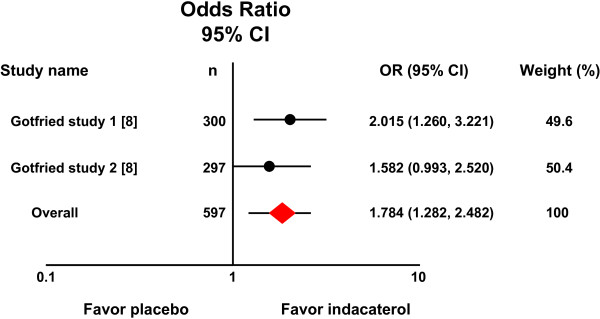
Forest plot of odds ratios and 95% confidence intervals. Relative to placebo, patients receiving indacaterol 75 μg once daily are more likely to achieve TDI score equal to or greater than one unit.
Indacaterol 150 μg versus placebo
Three randomized placebo-controlled trials compared indacaterol 150 μg once daily with placebo [5,7,9]. A meta-analysis that combined the three studies produced a pooled OR estimate of 2.149 (95% CI 1.746–2.645) with no evidence of heterogeneity (P = 0.686, I2 = 0.000), favoring patients who received indacaterol 150μg once daily. Figure 2 shows a forest plot of OR estimates from these studies.
Figure 2.
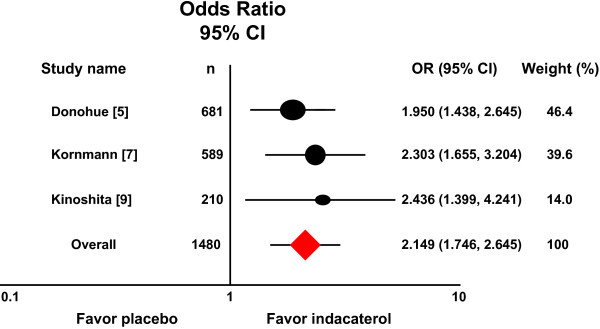
Meta-analysis of three randomized trials compared indacaterol 150 μg once daily with placebo. Relative to placebo, patients receiving indacaterol 150 μg once daily are more likely to achieve TDI score equal to or greater than one unit.
Indacaterol 300 μg versus placebo
Three trials compared indacaterol 300 μg once daily with placebo [5,6,9]. Figure 3 shows a forest plot of OR estimates from these studies. The combined OR estimate was 2.458 (95% CI 2.010–3.006) with no evidence of heterogeneity (P = 0.525, I2 = 0.000), again favoring patients who received indacaterol 300 μg once daily.
Figure 3.
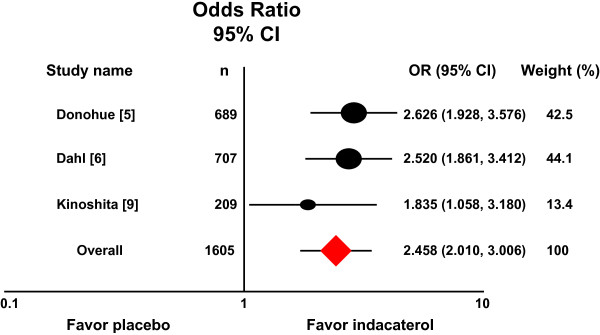
Meta-analysis of three randomized trials compared indacaterol 300 μg once daily with placebo. Relative to placebo, patients receiving indacaterol 300 μg once daily are more likely to achieve TDI score equal to or greater than one unit.
Discussion
The present meta-analyses combined data from existing randomized placebo-controlled trials, using number of patients achieving the minimum clinically important difference (MCID) for TDI score ≥1 as an outcome measure, and evaluated the efficacy of once-daily indacaterol on TDI scores in patients with stable COPD. A favorable effect was consistently obtained for indacaterol over placebo: 75 μg od OR 1.784 (95% CI 1.282 to 2.482); 150 μg od OR 2.149 (95% CI 1.746 to 2.645); and 300 μg od OR 2.458 (95% CI 2.010 to 3.006). A trend of increasing patient benefit was observed as indacaterol doses increased.
Recent guidelines recommend regular use of bronchodilators, such as indacaterol, as a long-term maintenance treatment of airflow limitation in COPD [1]. The extent to which indacaterol improves airflow in patients with COPD is well studied [4,5,21-24]. In these phase III studies, indacaterol provided 24-h bronchodilation on once-daily dosing with an effect that was sustained during treatment for up to one year. However, from the patients’ viewpoint, it may be more important to know whether this therapy improves dyspnea associated with daily activities, and these meta-analyses of available randomized placebo-controlled trials comparing indacaterol with placebo allows us to examine the efficacy of indacaterol on this important patient outcome. In all trials included in the meta-analyses, dyspnea was assessed using validated multidimensional instruments of BDI and TDI that are widely used to measure treatment effects in COPD [13-17]. When data were analyzed for a pre-determined outcome, i.e. the number of patients responding with a change of TDI equal to or greater than the minimum clinically important difference MCID (a ‘responder analysis’), a favorable effect was obtained for all three doses of indacaterol. The overall OR estimates of the meta-analyses are largely consistent with the results from the individual studies, in which the ORs for patients achieving a MCID in the indacaterol group compared with the placebo group varied from 1.582 to 2.015 for the 75 μg dose (Figure 1) [8], 1.950 to 2.303 for the 150 μg dose (Figure 2) [5,7], and 2.520 to 2.626 for the 300 μg dose (Figure 3) [5,6], with a exception of the Asian study [9]. It becomes apparent that patients receiving indacaterol had clinically significant improvements in symptoms of dyspnea compared to placebo over 12 weeks of treatment.
The overall odds ratio for response in TDI appeared to vary depending on indacaterol doses, and tended to increase with increasing indacaterol doses. Additionally, the percentage of patients who exceeded the one unit of the MCID in TDI varied in different indacaterol doses. The percentage in the analysis of indacaterol 75 μg once daily was 48%, compared with 34% in placebo. Compared to placebo, a higher percentage of patients achieved the MCID with the indacaterol 150 μg (60% versus 41%) and indacaterol 300 μg doses (63% versus 41%). Renard and his colleagues recently performed a model based analysis of the bronchodilatory dose response to indacaterol in patients with COPD [25]. The analysis demonstrated that indacaterol dosages of 75 μg once daily and above achieved minimal clinically important improvements in predicted trough FEV1 response, although dosages of 150 μg and 300 μg once daily provided optimum bronchodilation. The analysis also demonstrated that disease severity, as determined by FEV1, significantly affected dose response, suggesting that higher doses may be required in patients with more severe COPD to achieve optimal reduction of dyspnea.
In six trials included in the meta-analysis (n=5405), patients with COPD who received indacaterol 75 μg, or 150 μg, or 300 μg od had a significantly higher trough FEV1 than placebo after at least 12 weeks, with indacaterol increasing trough FEV1 by 120ml to 200 ml over placebo at week 12 [5-9]. The overall improvement in FEV1, though modest, may be sufficient to decrease the extent of hyperinflation which contributes to the sensation of dyspnea [26-29]. A better indicator of the effect of a bronchodilator on hyperinflation (and therefore the sensation of dyspnea) is perhaps inspiratory capacity. One could hypothesize that the prolonged bronchodilation observed with indacaterol would be associated with reductions of air trapping, and therefore reductions in hyperinflation, which would then be reflected in improvements in the sensation of dyspnea. There are relatively few indacaterol studies that included both an assessment of inspiratory capacity and of dyspnea, however, two short-term exercise studies comparing the 300 μg dose of indacaterol with placebo have been published [30,31]. The BDI/TDI was included in one of the studies, an improvement of 182 ml in resting inspiratory capacity after 14 days (p<0.05 vs. placebo) was associated with a change of TDI total score of +3.33 (p<0.01 vs. placebo) [31], providing indirect evidence to support the hypothesis.
Conclusions
Once daily indacaterol provides clinically significant improvements in dyspnea compared with placebo after 12 weeks of treatment. Incremental benefits in TDI were observed with increasing doses of indacaterol. Indacaterol may provide patients and physicians with a useful treatment option in symptomatic patients with dyspnea. Further studies, that investigate the relationship between disease severity as determined by FEV1 and dose response to indacaterol in terms of dyspnea, are needed to specifically address the question whether higher doses are required in patients with more severe COPD to achieve optimal reduction of dyspnea.
Abbreviations
BDI: Baseline dyspnea index; COPD: Chronic obstructive pulmonary disease; FEV1: Forced expiratory volume in 1 second; FVC: Forced vital capacity; GOLD: Global initiative for obstructive lung disease; MCID: Minimal clinically important difference; TDI: Transition dyspnea index; RCTs: Randomized controlled trials.
Competing interests
JNH has no conflict of interests to declare. LD is an employee of Novartis China. NSZ has no conflict of interests to declare.
Authors’ contributions
JNH, LD and NSZ were involved in the concept and design of this article and the interpretation of the data. JNH was responsible for analysis of data. All authors were participated in all stages of developing the manuscript. All authors revised the manuscript critically for important intellectual content, and gave their final approval of the version to be published.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Jiangna Han, Email: Janet_Han2000@hotmail.com.
Lu Dai, Email: lu.dai@novartis.com.
Nanshan Zhong, Email: nanshan@vip.163.com.
Acknowledgements
The authors thank David Young (Novartis, Horsham, West Sussex) for providing detailed information of various studies and for his help in the preparation of the manuscript.
This study was funded by National Science and Technology Infrastructure Program for the 12th Five-Year Plan.
References
- Global Initiative for Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2011. http://www.goldcopd.org/Guidelines/guidelines-gold-summary-2011.html.
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, MacDonald R, Shekelle P. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri-Kohlman V, Curtis JR, Manning HL, Mularski RA, Varkey B, Campbell M, Carter ER, Chiong JR, Ely EW, Hansen-Flaschen J, O’Donnell DE, Waller A. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137:674–691. doi: 10.1378/chest.09-1543. [DOI] [PubMed] [Google Scholar]
- Balint B, Watz H, Amos C, Owen R, Higgins M, Kramer B. Onset of action of indacaterol in patients with COPD: Comparison with salbutamol and salmeterol-fluticasone. Int J Chron Obstruct Pulmon Dis. 2010;5:311–318. doi: 10.2147/copd.s12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue JF, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorgancioglu A, Iqbal A, Swales J, Owen R, Higgins M, Kramer B. Once-daily bronchodilators for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:155–162. doi: 10.1164/rccm.200910-1500OC. [DOI] [PubMed] [Google Scholar]
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Bleasdale P, Owen R, Higgins M, Kramer B. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65:473–479. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, Kramer B. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37:273–279. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- Gotfried MH, Kerwin EM, Lawrence D, Lassen C, Kramer B. Efficacy of indacaterol 75 μg once-daily on dyspnea and health status: results of two double-blind, placebo-controlled 12-week studies. COPD. 2012;9:1–8. doi: 10.3109/15412555.2012.651935. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Lee SH, Hang LW, Ichinose M, Hosoe M, Okino N, Prasad N, Kramer B, Fukuchi Y. Efficacy and safety of indacaterol 150 and 300 μg in chronic obstructive pulmonary disease patients from six Asian areas including Japan: a 12-week, placebo-controlled study. Respirology. 2012;17:379–389. doi: 10.1111/j.1440-1843.2011.02107.x. [DOI] [PubMed] [Google Scholar]
- Kerwin EM, Gotfried MH, Lawrence D, Lassen C, Kramer B. Efficacy and tolerability of indacaterol 75 μg once daily in patients aged ≥40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week studies. Clin Ther. 2011;33:1974–1984. doi: 10.1016/j.clinthera.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, Fein A, Heffner J, Lareau S, Meek P, Martinez F, McNicholas W, Muris J, Austegard E, Pauwels R, Rennard S, Rossi A, Siafakas N, Tiep B, Vestbo J, Wouters E, ZuWallack R. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2005. http://www.goldcopd.org/search.html?q=GOLD+2005+update&user.
- Jones P, Lareau S, Mahler DA. Measuring the effects of COPD on the patient. Respir Med. 2005;99(Suppl B):S11–S18. doi: 10.1016/j.rmed.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- Witek TJ Jr, Mahler DA. Meaningful effect size and patterns of response of the transition dyspnea index. J Clin Epidemiol. 2003;56:248–255. doi: 10.1016/S0895-4356(02)00589-9. [DOI] [PubMed] [Google Scholar]
- Witek TJ Jr, Mahler DA. Minimal important difference of the transition dyspnea index in a multinational clinical trial. Eur Respir J. 2003;21:267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Witek TJ Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2:99–103. doi: 10.1081/COPD-200050666. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Mosteller F, Colditz GA. Understanding research synthesis (meta-analysis) Annu Rev Publ Health. 1996;17:1–23. doi: 10.1146/annurev.pu.17.050196.000245. [DOI] [PubMed] [Google Scholar]
- Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167:335–342. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, Lawrence D. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulm Pharmacol Ther. 2010;23:165–171. doi: 10.1016/j.pupt.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, Ramos-Barbon D, Jack D, Piggott S, Owen R, Higgins M, Kramer B. Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium. Respir Res. 2010;11:135. doi: 10.1186/1465-9921-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnussen H, Verkindre C, Jack D, Jadayel D, Henley M, Woessner R, Higgins M, Kramer B. Indacaterol once-daily is equally effective dosed in the evening or morning in COPD. Respir Med. 2010;104:1869–1876. doi: 10.1016/j.rmed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B. Long-term safety and efficacy of indacaterol, a long-acting ß2-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest. 2011;140:68–75. doi: 10.1378/chest.10-1830. [DOI] [PubMed] [Google Scholar]
- Renard D, Looby M, Kramer B, Lawrence D, Morris D, Stanski DR. Characterization of the bronchodilatory dose response to indacaterol in patients with chronic obstructive pulmonary disease using model-based approaches. Respir Res. 2011;12:54. doi: 10.1186/1465-9921-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CB. Airflow obstruction and exercise. Respir Med. 2009;103:325–334. doi: 10.1016/j.rmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Donohue JF. Pharmacologic interventions in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:526–534. doi: 10.1513/pats.200701-016FM. [DOI] [PubMed] [Google Scholar]
- Malerba M, Radaeli A, Morjaria JB. Therapeutic potential for novel untra long-acting ß2-agonists in the management of COPD: biological and pharmacological aspects. Drug Discov Today. 2012;17:496–504. doi: 10.1016/j.drudis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Fuso L, Mores N, Valente S, Malerba M, Montuschi P. Long-acting beta-agonists and their association with inhaled corticosteroids in COPD. Curr Med Chem. 2013. Epub ahead of print. [DOI] [PubMed]
- O’Donnell DE, Casaburi R, Vincken W, Puente-Maestu L, Swales J, Lawrence D, Kramer B. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med. 2011;105:1030–1036. doi: 10.1016/j.rmed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Wagner F, Khindri S, Drollmann AF. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD. 2011;8:340–345. doi: 10.3109/15412555.2011.594464. [DOI] [PubMed] [Google Scholar]


