Abstract
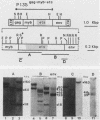
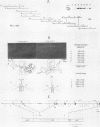
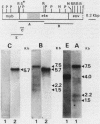
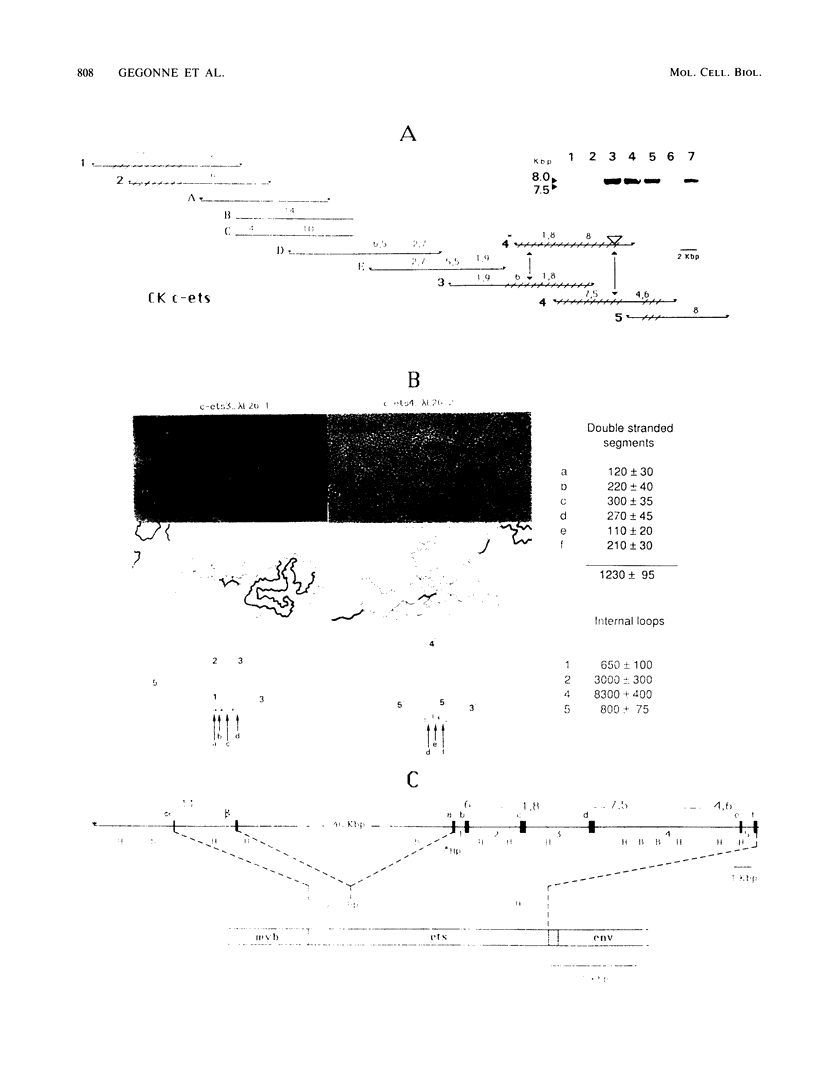
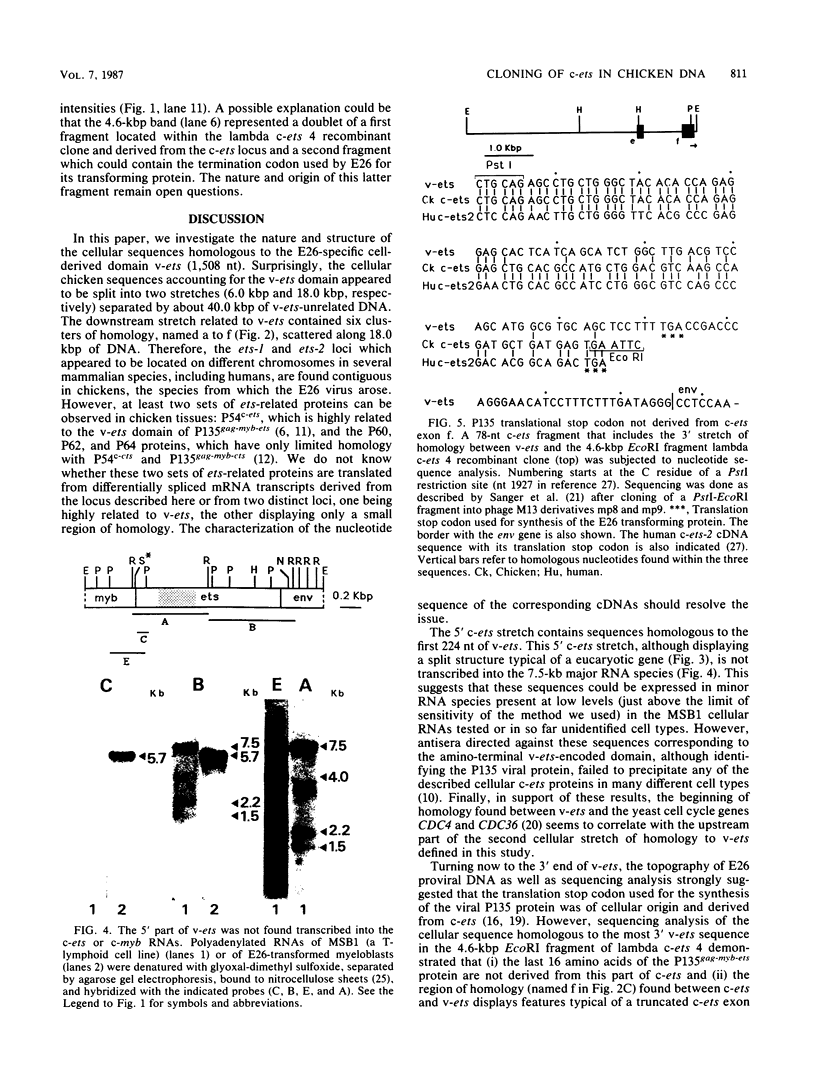
We have investigated the structure of chicken genomic DNA homologous to v-ets, the second cell-derived oncogene of avian retrovirus E26. We isolated a c-ets locus spanning ca. 30.0 kilobase pairs (kbp) in the chicken genome with homologies to 1,202 nucleotides (nt) of v-ets (total length, 1,508 nt) distributed in six clusters along 18.0 kbp of the cloned DNA. The 5'-distal part of v-ets (224 nt) was homologous to chicken cellular sequences contained upstream within a single 16.0-kbp EcoRI fragment as two typical exons but not found transcribed into the major 7.5-kb c-ets (or 4.0-kb c-myb) RNA species. Between these two v-ets-related cellular sequences we found ca 40.0 kbp of v-ets-unrelated DNA. Finally, the most 3' region of homology to v-ets in the cloned DNA was shown to consist of a truncated exon lacking the nucleotides coding for the 16 carboxy-terminal amino acids of the viral protein but colinear to one of the two human c-ets loci, c-ets-2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Graf T. Myeloblasts transformed by the avian acute leukemia virus E26 are hormone-dependent for growth and for the expression of a putative myb-containing protein, p135 E26. EMBO J. 1982;1(9):1069–1073. doi: 10.1002/j.1460-2075.1982.tb01298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Bister K., Nunn M., Moscovici C., Perbal B., Baluda M., Duesberg P. H. Acute leukemia viruses E26 and avian myeloblastosis virus have related transformation-specific RNA sequences but different genetic structures, gene products, and oncogenic properties. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3677–3681. doi: 10.1073/pnas.79.12.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Lampert M. A., Lipsick J. S., Baluda M. A. Avian myeloblastosis virus and E26 virus oncogene products are nuclear proteins. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4265–4269. doi: 10.1073/pnas.81.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. The proto-oncogene c-ets is preferentially expressed in lymphoid cells. Mol Cell Biol. 1985 Nov;5(11):2993–3000. doi: 10.1128/mcb.5.11.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll J., Righi M., Taisne C., Dissous C., Gegonne A., Stehelin D. Molecular cloning of the avian acute transforming retrovirus MH2 reveals a novel cell-derived sequence (v-mil) in addition to the myc oncogene. EMBO J. 1983;2(12):2189–2194. doi: 10.1002/j.1460-2075.1983.tb01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Gegonne A., Pognonec P., Leprince D., Dernis D., Remaut E., Stehelin D., Ghysdael J. Préparation et caractérisation d'antisera specifiques de différents domaines polypeptidiques correspondant à l'oncogène v-ets du virus aviaire de leucémies aiguës E26. C R Acad Sci III. 1986;303(7):253–256. [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Boulukos K., Leprince D., Dernis D., Lagrou C., Stehelin D. Identification in chicken macrophages of a set of proteins related to, but distinct from, the chicken cellular c-ets-encoded protein p54c-ets. EMBO J. 1986 Sep;5(9):2251–2256. doi: 10.1002/j.1460-2075.1986.tb04492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Gegonne A., Pognonec P., Dernis D., Leprince D., Stehelin D. Identification and preferential expression in thymic and bursal lymphocytes of a c-ets oncogene-encoded Mr 54,000 cytoplasmic protein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1714–1718. doi: 10.1073/pnas.83.6.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Stéhelin D. Avian leukemia viruses. Oncogenes and genome structure. Biochim Biophys Acta. 1982 Jun 28;651(4):245–271. doi: 10.1016/0304-419x(82)90014-2. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Franchini G., Love J., Simon M. I., Gallo R. C., Wong-Staal F. Chromosomal sublocalization of human c-myb and c-fes cellular onc genes. Nature. 1983 Jul 14;304(5922):169–171. doi: 10.1038/304169a0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Leprince D., Gegonne A., Coll J., de Taisne C., Schneeberger A., Lagrou C., Stehelin D. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983 Nov 24;306(5941):395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Peterson T. A., Yochem J., Byers B., Nunn M. F., Duesberg P. H., Doolittle R. F., Reed S. I. A relationship between the yeast cell cycle genes CDC4 and CDC36 and the ets sequence of oncogenic virus E26. Nature. 1984 Jun 7;309(5968):556–558. doi: 10.1038/309556a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saule S., Coll J., Righi M., Lagrou C., Raes M. B., Stehelin D. Two different types of transcription for the myelocytomatosis viruses MH2 and CMII. EMBO J. 1983;2(6):805–809. doi: 10.1002/j.1460-2075.1983.tb01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saule S., Sergeant A., Torpier G., Raes M. B., Pfeifer S., Stehelin D. Subgenomic mRNA in OK10 defective leukemia virus-transformed cells. J Virol. 1982 Apr;42(1):71–82. doi: 10.1128/jvi.42.1.71-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams-Smith M. J., Kozak C., Reeves R., Gearhart J., Nunn M. F., Nash W., Fowle J. R., 3rd, Duesberg P., Papas T. S. Conserved chromosomal positions of dual domains of the ets protooncogene in cats, mice, and humans. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1792–1796. doi: 10.1073/pnas.83.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams-Smith M. J., Nunn M. F., Duesberg P. H., O'Brien S. J., Papas T. S. The ets sequence from the transforming gene of avian erythroblastosis virus, E26, has unique domains on human chromosomes 11 and 21: both loci are transcriptionally active. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7294–7298. doi: 10.1073/pnas.82.21.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Taisne C., Gegonne A., Stehelin D., Bernheim A., Berger R. Chromosomal localization of the human proto-oncogene c-ets. Nature. 1984 Aug 16;310(5978):581–583. doi: 10.1038/310581a0. [DOI] [PubMed] [Google Scholar]