Abstract
Rhomboid domain-containing protein 3 (Rhbdd3), which belongs to a family of proteins with rhomboid domain, is widely expressed in immune cells; however, the roles of the Rhbdd members, including Rhbdd3, in immunity remain unknown. Natural killer (NK) cells are critical for host immune defense and also can mediate inflammatory diseases such as hepatitis. Although much is known about how NK cells are activated, the detailed mechanisms for negative regulation of NK cell activation remain to be fully understood. Using Rhbdd3-deficient mice, we reveal that Rhbdd3, selectively up-regulated in NK cells upon Toll-like receptor 3 (TLR3) stimulation, negatively regulates TLR3-mediated NK cell activation in a feedback manner. Rhbdd3 inhibits TLR3-triggered IFN-γ and granzyme B expression of NK cells in cell–cell contact dependence of accessory cells such as dendritic cells and Kupffer cells. Rhbdd3 interacts with DNAX activation protein of 12 kDa and promotes its degradation, inhibiting MAPK activation in TLR3-triggered NK cells. Furthermore, Rhbdd3 plays a critical role in attenuating TLR3-triggered acute inflammation by controlling NK cell activation and accumulation in liver and disrupting NK cell–Kupffer cell interaction. Therefore, Rhbdd3 is a feedback inhibitor of TLR3-triggered NK cell activation. Our study outlines a mechanism for the negative regulation of NK cell activation and also provides clues for the function of the rhomboid proteins in immunity.
Keywords: poly(I:C), innate immunity, immune regulation
Natural killer (NK) cells are essential for innate host defense against viral infection and tumor via releasing cytotoxic granules and cytokines, as well as lysis of infected or transformed cells (1). NK cells also function as regulatory cells through interacting with various components of the immune system (2, 3). Dysfunction of NK cells has been linked to the pathogenesis of inflammatory and autoimmune diseases with particular functional involvement in liver inflammation and injury during viral hepatitis (4, 5). Thus, identification of new regulators of NK cell activation will contribute to a better understanding of how to maintain immune homeostasis and may provide potential targets for prevention and treatment of inflammatory diseases resulting from aberrant NK cell activation.
Activation of NK cells is finely regulated by a series of surface-activating or inhibitory receptors, the balance between which sets a threshold for NK cell activation (6). Besides, NK cells express many types of Toll-like receptors (TLRs) and can be activated by various TLR ligands. For example, TLR4 ligand LPS, TLR7/8 ligand R-848, and TLR9 ligand CpG induce NK cell activation, either directly or indirectly (7–9). Notably, human NK cells express the highest level of TLR3 among major immune cells (10). Double-stranded (ds) RNA polyinosinic-polycytidylic acid [poly(I:C)] activates NK cells through recognition by TLR3 (11). TLR3 triggers the recruitment of TRIF (Toll-interleukin 1 receptor domain-containing adapter inducing IFN-β), leading to the activation of downstream mitogen-activated protein kinase (MAPK) family and nuclear factor κB (NF-κB) and IFN-regulatory factor 3 transcription factors (12). Moreover, NK cells can be activated by cytokines such as IL-12 and IL-15 through the signal transducer and activator of transcription (STAT) pathway (13, 14). Although much is known about how NK cells are activated, the detailed mechanisms for the negative regulation of NK cell activation remain to be fully understood.
Growing evidence shows that TLR3 is important in the pathogenesis of a variety of liver diseases (15). Poly(I:C) injection in vivo induces massive liver damage in D-galactosamine (D-GalN)–sensitized mice mediated by a crosstalk between NK cells and Kupffer cells (KCs) (16). Thus, the investigation into how NK cell function is regulated, herein with the model of acute liver inflammation, will contribute to better understand how the disordered NK cells are involved in the pathogenesis during the innate inflammatory response and how to control NK cell activation for the maintenance of immune homeostasis.
Rhomboids are a family of widely distributed intramembrane serine proteases involved in growth factor signaling, mitochondrial remodeling, and apoptosis (17). Rhomboids are also associated with various human diseases (18). Rhomboid domain-containing protein (Rhbdd) belongs to a family of proteins with rhomboid domain. To date, there are only a few reports of Rhbdd1, Rhbdd2, and Rhbdd3 (also known as pituitary tumor apoptosis gene) that indicate their roles in apoptosis and cancer (19–21). Interestingly, inactive rhomboid protein 2 has been more recently shown to facilitate LPS-induced TNF production (22, 23), highlighting the involvement of rhomboid-like proteins in immunological processes. However, to date, there is no report about the roles of Rhbdd proteins in immunity. On the basis of the preliminary observation that Rhbdd3 is selectively up-regulated in TLR3-activated NK cells, here we generated Rhbdd3-deficient mice and investigated the role of Rhbdd3 in NK cell responses. We demonstrate that Rhbdd3 negatively regulates TLR3-triggered acute inflammation by controlling NK cell activation, providing insights into how NK cell activation by TLR3 is controlled and important clues for the function of rhomboids protein in immunity.
Results
Rhbdd3 Negatively Regulates TLR3-Triggered NK Cell Activation in Vitro and in Vivo.
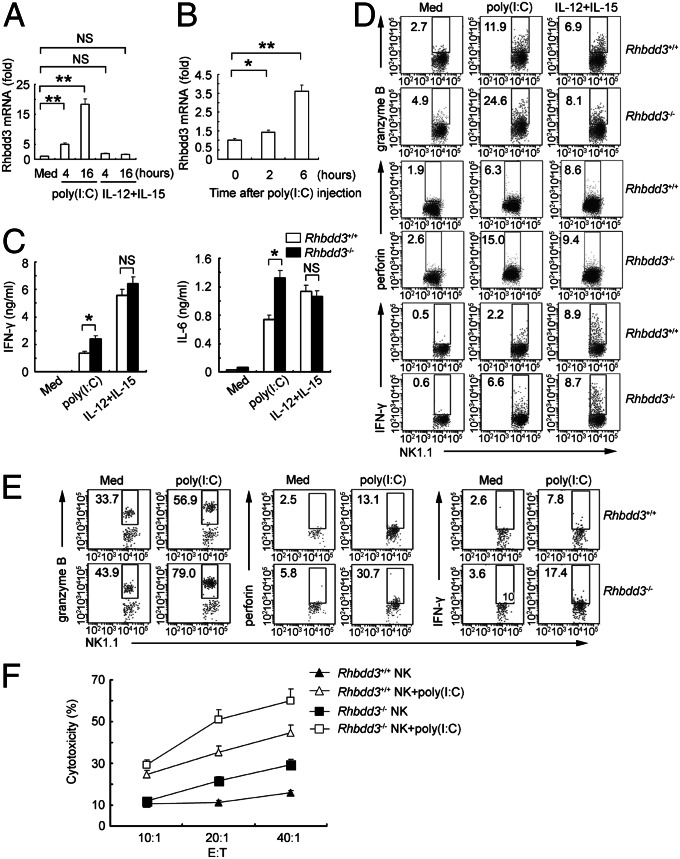
We first analyzed the dynamic expression of Rhbdd3 during NK cell activation. TLR3 agonists poly(I:C) significantly increased Rhbdd3 expression in NK cells, whereas IL-12/15 did not affect its expression (Fig. 1A). Consistently, Rhbdd3 increased quickly and significantly in NK cells after in vivo injection of poly(I:C) (Fig. 1B). Therefore, Rhbdd3 is significantly up-regulated by TLR3 stimulation in NK cells.
Fig. 1.
Rhbdd3 negatively regulates TLR3-induced NK cell activation in vitro and in vivo. (A) Splenocytes were stimulated with poly(I:C) or IL-12/15. NK cells were purified then and assessed for Rhbdd3 mRNA expression using quantitative PCR. (B) Splenic NK cells collected from mice i.p. injected with poly(I:C) and D-GalN were assessed for Rhbdd3 mRNA expression using quantitative PCR. (C and D) Rhbdd3+/+ and Rhbdd3−/− splenocytes were stimulated with medium (Med), poly(I:C), or IL-12/15. The levels of IFN-γ and IL-6 in supernatants (C) and intracellular expression of granzyme B, perforin, and IFN-γ in NK1.1+ cells (D) were, respectively, measured by cytometric bead array (CBA) and FACS. (E and F) Rhbdd3+/+ and Rhbdd3−/− mice (six mice per group) were i.p. injected with poly(I:C) and D-GalN. After 6 h, the liver mononuclear cells were collected. The intracellular granzyme B, perforin, and IFN-γ in liver NK1.1+ cells were determined by FACS (E). Liver NK cells were further isolated and assessed for their cytotoxicity against YAC-1 cells. E:T, effector versus target (F). The data shown are the means ± SD from three independent experiments (A–C and F). *P < 0.05; **P < 0.01; NS, not significant.
To study the immunological role of Rhbdd3 in vivo, we generated Rhbdd3-dificient (Rhbdd3−/−) mice through homologous recombination. The distributions of B cells, T cells, and NK cells, as well as myeloid cells, were comparable between Rhbdd3+/+ and Rhbdd3−/− mice, suggesting that Rhbdd3 does not affect the development of these immune cells (Fig. S1). We then investigated whether the inducible Rhbdd3 may feedback-regulate TLR3-mediated NK cell activation. Rhbdd3−/− splenocytes produced higher levels of IFN-γ and IL-6 upon poly(I:C) stimulation than Rhbdd3+/+ splenocytes but similar levels of IFN-γ and IL-6 upon IL-12/15 stimulation (Fig. 1C). Importantly, the intracellular expression of granzyme B, perforin, and IFN-γ in NK1.1+ cells upon poly(I:C) stimulation was also higher in Rhbdd3−/− splenocytes, still with no obvious difference after IL-12/15 stimulation (Fig. 1D and Fig. S2A). Besides, neither NK cell percentage in spleen (Fig. S2B) nor TLR3 expression on NK cells (Fig. S2C) differed between Rhbdd3+/+ and Rhbdd3−/− mice, indicating that the elevated cytokines from TLR3-activated Rhbdd3−/− splenocytes were attributable to strengthened NK cell activation rather than the elevated NK cell proportion or TLR3 expression of NK cells. Thus, Rhbdd3 inhibits TLR3-triggered NK cell activation in vitro.
We next investigated whether Rhbdd3 also regulated NK cell activation in vivo during acute liver inflammation. Rhbdd3−/− liver NK cells expressed more granzyme B, perforin, and IFN-γ (Fig. 1E and Fig. S2D) and had stronger cytotoxicity against YAC-1 cells (Fig. 1F) after in vivo injection of poly(I:C). Thus, Rhbdd3 negatively regulates NK cell activation via TLR3 both in vitro and in vivo. Furthermore, Rhbdd3−/− mice exhibited an increased proportion of NK cells in liver after poly(I:C) challenge (Fig. S3), suggesting Rhbdd3 also affects NK cell accumulation in liver.
Accessory Cells (Dendritic Cells and KCs) Are Required for the Suppression of TLR3-Triggered NK Cell Activation by Rhbdd3 in a Cell–Cell Contact-Dependent Manner.
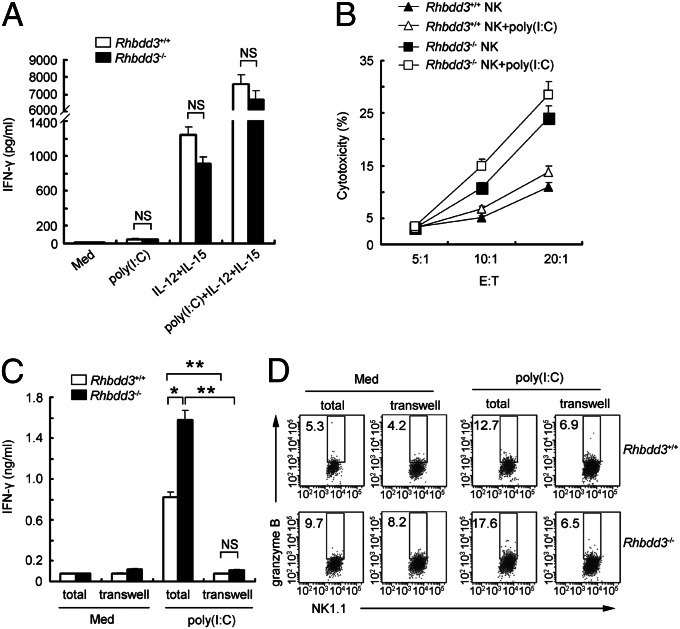
We then isolated splenic DX5+ NK cells to investigate the direct involvement of Rhbdd3 in TLR3-triggered NK cell activation. Unexpectedly, poly(I:C) alone did not increase IFN-γ production by NK cells (Fig. 2A). After IL-12 and -15 were added to support NK cell survival, Rhbdd3+/+ and Rhbdd3−/− NK cells secreted a striking amount of IFN-γ upon poly(I:C) stimulation, nevertheless, at similar levels (Fig. 2A). Thus, TLR3 activates NK cells dependent on the presence of proinflammatory cytokines, and Rhbdd3 could not directly inhibit IFN-γ production by TLR3-activated NK cells dependent on IL-12/15. Moreover, Rhbdd3+/+ and Rhbdd3−/− NK cells produced similar levels of IFN-γ and IL-10 upon stimuli with LPS, CpG, IFN-α, or IL-2/12, which, unlike poly(I:C), could independently induce NK activation (Fig. S4A). Thus, Rhbdd3 is involved neither in NK cell activation by those signals nor in the regulatory function of NK cells. Interestingly, Rhbdd3−/− NK cells were more cytotoxic than Rhbdd3+/+ NK cells (Fig. 2B), indicating that Rhbdd3 inhibits NK cell cytotoxicity constitutively. We next wondered whether accessory cells in spleen could support the inhibitory effect of Rhbdd3 on NK cell activation. To answer this, we separated DX5+ and the rest DX5− part of splenocytes in a Transwell system (BD Falcon). Interestingly, the elevated production of IFN-γ and granzyme B by Rhbdd3−/− splenic NK cells upon poly(I:C) stimulation was dramatically reduced in the Transwell system, to a level similar to Rhbdd3+/+ mice (Fig. 2 C and D and Fig. S4B). Therefore, Rhbdd3 inhibits TLR3-mediated NK cell activation via accessory cells contacting with NK cells.
Fig. 2.
Accessory cells are required for the suppression of TLR3-triggered NK cell activation by Rhbdd3 in a cell–cell contact-dependent manner. (A) Rhbdd3+/+ and Rhbdd3−/− splenic NK cells were stimulated with poly(I:C), IL-12/15, or poly(I:C) plus IL-12/15, and IFN-γ levels in supernatants were assessed by CBA. (B) Rhbdd3+/+ and Rhbdd3−/− splenic NK cells were stimulated with poly(I:C) and then cultured with 5,6-carboxyfluorescein diacetate succinimidyl ester-labeled Yac-1 cells at indicated E:T ratios. The cytotoxicity of NK cells against YAC-1 cells were analyzed by FACS. (C and D) Splenic DX5+ NK cells and the left DX5− splenocytes, which were, respectively, seeded in the upper and lower compartments of the Transwell system (transwell), or total splenocytes (total) were stimulated with poly(I:C). IFN-γ levels in supernatants (C) and intracellular granzyme B in NK1.1+ cells (D) were respectively assessed by CBA and FACS. The data shown are the means ± SD (A–C) from three independent experiments. *P < 0.05; **P < 0.01; NS, not significant.
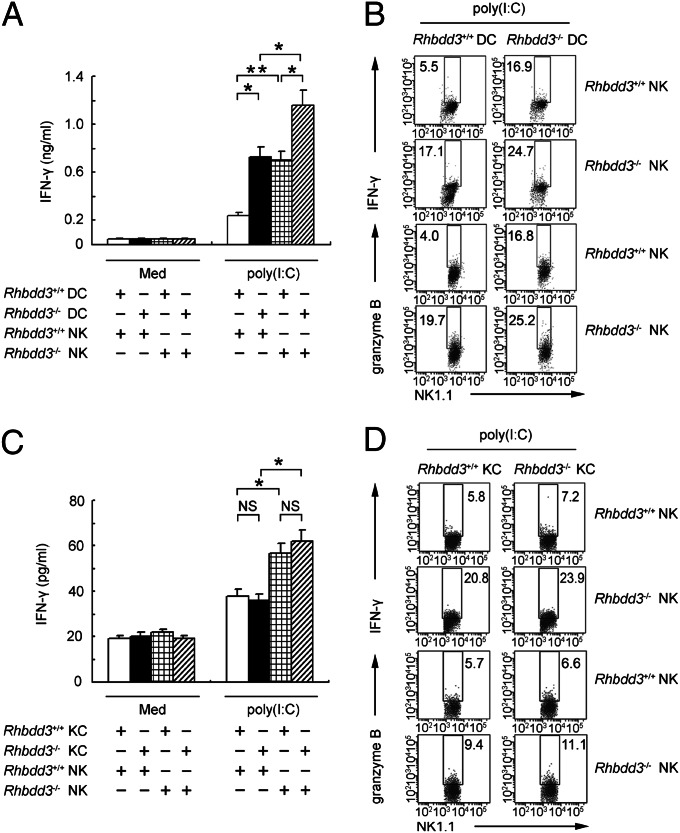
NK cells and dendritic cells (DCs) interact with each other reciprocally in a cell–cell contact-dependent manner (24), so we wondered whether DCs are involved in the suppressive effect of Rhbdd3 on TLR3-mediated NK cell activation. We stimulated Rhbdd3+/+ or Rhbdd3−/− NK cells with poly(I:C) in the presence of Rhbdd3+/+ or Rhbdd3−/− DCs. Poly(I:C) induced higher expression of IFN-γ and granzyme B (Fig. 3 A and B and Fig. S5A) in Rhbdd3−/− NK cells than Rhbdd3+/+ NK cells in the presence of DCs. Thus, Rhbdd3 inhibits TLR3-triggered NK cell activation in a DC-dependent manner. Interestingly, Rhbdd3−/− DCs also mediated elevated activation of NK cells by poly(I:C) stimulation than Rhbdd3+/+ DCs (Fig. 3 A and B), indicating the potential role of Rhbdd3 in regulating DC function. However, as shown in Fig. S5B, poly(I:C)-pretreated DCs alone could not induce NK cell activation in the absence of poly(I:C). Only under the continuous presence of poly(I:C) could poly(I:C)-pretreated DCs mediate NK cell activation, indicating that simultaneous presence of DCs and poly(I:C) are required for NK cell activation. In summary, negative regulation of poly(I:C)-induced NK cell activation by Rhbdd3 requires the presence of either naïve or poly(I:C)-pretreated DCs.
Fig. 3.
DCs and KCs are required for the suppression of NK cell activation by Rhbdd3. Rhbdd3+/+ or Rhbdd3−/− splenic NK cells were cocultured with Rhbdd3+/+ or Rhbdd3−/− bone marrow-derived (BM) DCs (A and B) or liver KCs (C and D) at a ratio of 5:1 and stimulated with poly(I:C). IFN-γ levels in supernatants (A and C) and intracellular production of IFN-γ and granzyme B in NK1.1+ cells (B and D) were, respectively, assessed using CBA or FACS. The data shown are the means ± SD (A and C) from three independent experiments. *P < 0.05; **P < 0.01; NS, not significant.
A crosstalk between NK cells and KCs in liver are critically pathogenic factors in TLR3-triggered liver inflammation (16). Similarly, the expression of IFN-γ and granzyme B (Fig. 3 C and D and Fig. S5C) were significantly increased in TLR3-activated Rhbdd3−/− NK cells in the presence of KCs than that in Rhbdd3+/+ NK cells, indicating that KCs are also involved in TLR3-triggered NK cell activation and that Rhbdd3 negatively regulates TLR3-triggered NK cell activation dependent on the presence of KCs.
Rhbdd3 Negatively Regulates NK Cell Activation by Promoting DNAX Activation Protein of 12 kDa Degradation and Inhibiting MAPK Activation by TLR3 Stimulation.
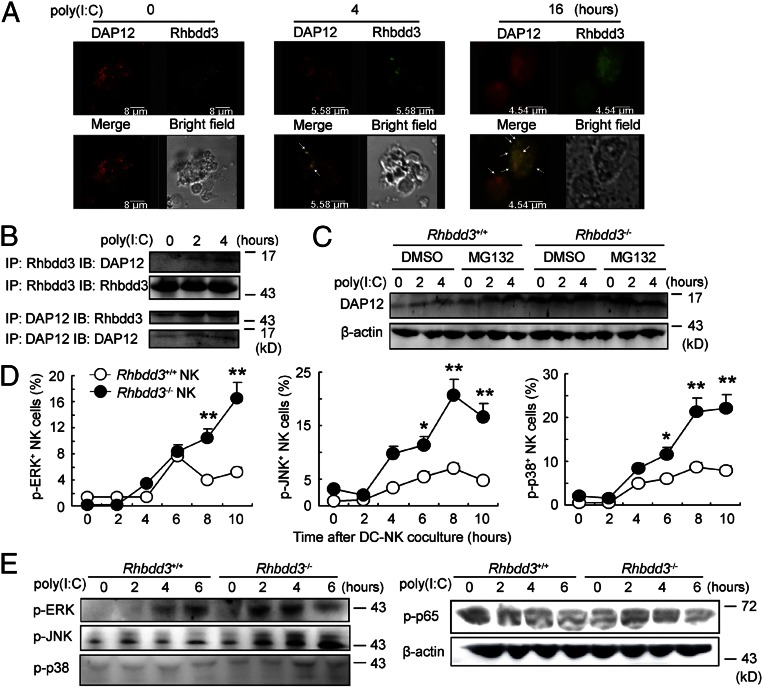
The above data suggest that Rhbdd3 inhibits TLR3-mediated NK cell activation via accessory cells such as DCs and KCs. NK cell activation is determined by the balance between activating receptors and inhibitory receptors; however, no significant difference in natural-killer group 2 member D, lymphocyte antigen 49D, natural-killer group 2 member A and lymphocyte antigen 49A expression on NK cells was observed between Rhbdd3−/− and Rhbdd3+/+ mice (Fig. S6). DNAX activation protein of 12 kDa (DAP12) is a key intracellular accessory adaptor of several activating receptors on NK cells (25). Therefore, we next explored whether Rhbdd3 is involved in the regulation of DAP12. Through confocal analysis, we found that DAP12 and Rhbdd3 increased and aggregated significantly after poly(I:C) stimulation (Fig. 4A). Coimmunoprecipitation analysis showed that Rhbdd3 interacted with DAP12 in poly(I:C)-activated NK cells (Fig. 4B). Furthermore, the expression of DAP12 at protein level, but not at mRNA level, after TLR3 stimulation was significantly elevated by Rhbdd3 knockdown or depletion (Fig. 4C and Fig. S7 A and B). Proteasome inhibitor MG132 pretreatment increased the DAP12 expression in Rhbdd3+/+ NK cells but not in Rhbdd3−/− NK cells (Fig. 4C). Therefore, Rhbdd3 can promote proteasomal degradation of DAP12 and, thus, inhibit TLR3-triggered DAP12 expression.
Fig. 4.
Inhibition of TLR3-triggered NK cell DAP12 expression and MAPK activation by Rhbdd3. (A) Splenocytes were stimulated with poly(I:C) for the indicated time, and then NK cells were isolated. The intracellular localization of Rhbdd3 (red) and DAP12 (green) were determined by confocal analysis. (Objective, 40×; numerical aperture, 1.4.) Bar lengths are as indicated. (B) Cell lysates from poly(I:C)-activated splenic NK cells were immunoprecipitated and immunoblotted by anti-Rhbdd3 antibody or anti-DAP12 antibody as indicated. (C) Rhbdd3+/+ or Rhbdd3−/− splenic NK cells were treated with MG132 (20 μM) or DMSO (vehicle control) for 2 h and then stimulated by poly(I:C) for the indicated time. The expression of DAP12 was analyzed by Western blotting. (D) Rhbdd3+/+ or Rhbdd3−/− splenic NK cells were stimulated with poly(I:C) in the presence of BMDCs. The levels of p-ERK, p-JNK, and p-p38 in NK1.1+ NK cells were determined using a Phosflow method (BD Biosciences). (E) Splenic NK cells were, respectively, purified from Rhbdd3+/+ and Rhbdd3−/− mice i.p. treated with poly(I:C) and D-GalN. The expression of p-ERK, p-JNK, p-p38, and p-p65 were evaluated by Western blotting. The data shown are the means ± SD (D) from three independent experiments. *P < 0.05; **P < 0.01; NS, not significant.
DAP12-associated activating receptors may induce activation of downstream signaling molecules including MAPK and NF-κB (6, 25). As shown in Fig. 4D, the levels of p-ERK, p-JNK, and p-protein 38 (p-p38) by TLR3 stimulation were significantly higher in Rhbdd3−/− NK cells than Rhbdd3+/+ NK cells in the presence of DCs. Moreover, the activation of p-ERK, p-JNK, and p-p38 was consistently higher in Rhbdd3−/− splenic NK cells after in vivo poly(I:C) stimulation, whereas the activation of NF-κB was similar (Fig. 4E). Together, Rhbdd3 negatively controls TLR3 signaling in NK cells by promoting DAP12 degradation and inhibiting MAPK activation.
Rhbdd3 Attenuates TLR3-Triggered Acute Liver Inflammation Through Controlling NK Cell Activation and NK Cell–KC Crosstalk.
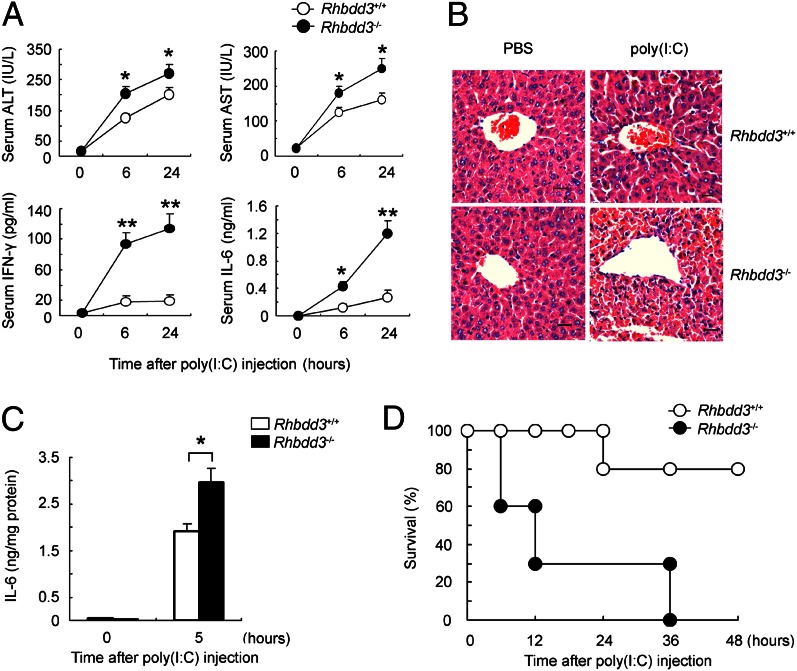
Because Rhbdd3 inhibits TLR3-triggered NK cell activation and accumulation in liver, we next explored the biological function of Rhbdd3 in vivo. We induced acute liver inflammation through injection of poly(I:C) and D-GalN. Rhbdd3−/− mice exhibited a more exaggerated elevation of serum alanine transaminase (ALT), aspartate transaminase (AST), IFN-γ, and IL-6 than Rhbdd3+/+ mice after poly(I:C) injection (Fig. 5A). Accordingly, hepatic pathology revealed significant increases in inflammatory infiltrates and necrosis in Rhbdd3−/− mice (Fig. 5B). Moreover, we found more IL-6 in liver (Fig. 5C) and accelerated death (Fig. 5D) in Rhbdd3−/− mice. Thus, Rhbdd3 prevents the development of TLR3-mediated acute liver inflammation.
Fig. 5.
Rhbdd3 attenuates poly(I:C)-induced acute liver inflammation. (A–D) Rhbdd3+/+ and Rhbdd3−/− mice (six mice per group) were i.p. injected with poly(I:C) and D-GalN. (A) The levels of ALT, AST, IFN-γ, and IL-6 in serum were, respectively, assayed using ELISA (ALT and AST) and CBA (IFN-γ and IL-6). (B and C) After 6 h, liver tissues were collected. The pathology of liver was analyzed by microscopy. [Original magnification, 400×; bars, 10 μm (B).] IL-6 levels in liver homogenates were determined by CBA and then normalized against milligrams of total protein determined by BCA assay (C). (D) The survival rate of Rhbdd3+/+ and Rhbdd3−/− mice (10 mice per group) was monitored for 48 h. P < 0.01 by Wilcoxon test. The data shown are the means ± SD (A and C) from three independent experiments. *P < 0.05; **P < 0.01.
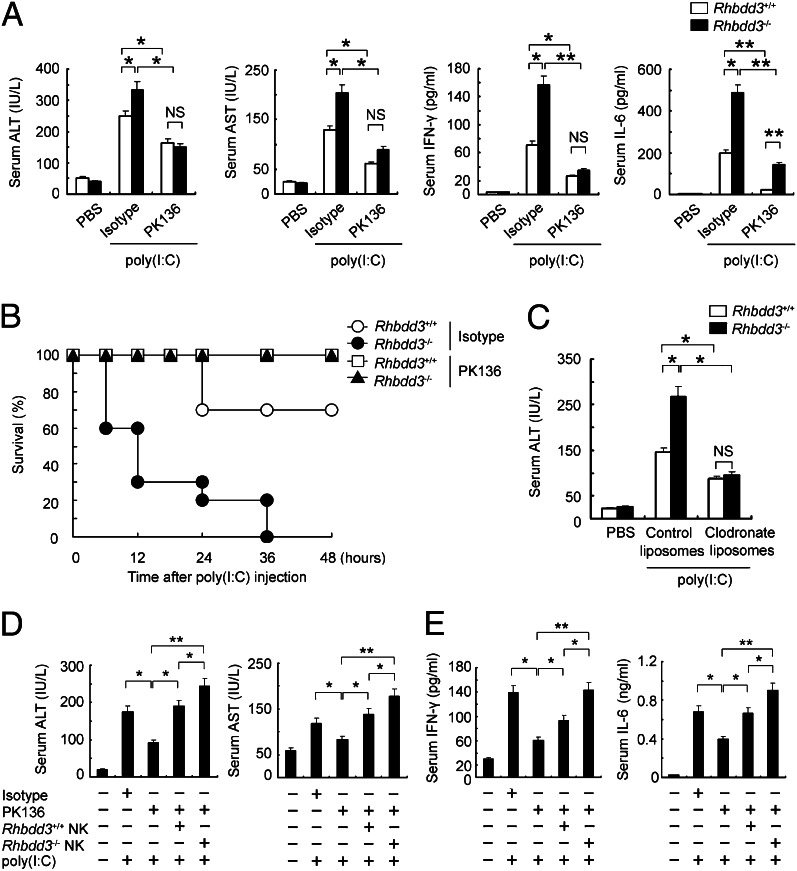
Our previous work demonstrated that NK cells are responsible for the pathogenesis of poly(I:C)-induced acute liver inflammation (26). Therefore, we next wondered whether Rhbdd3 attenuates poly(I:C)-induced acute liver inflammation through affecting NK cell activation. We depleted NK cells through administration of monoclonal antibody PK136 against mouse NK1.1 antigen before poly(I:C) injection. As shown in Fig. 6A, depletion of NK cells significantly prevented both Rhbdd3+/+ and Rhbdd3−/− mice from liver inflammation. Moreover, the levels of AST and ALT were similar between NK cell-depleted Rhbdd3+/+ and Rhbdd3−/− mice after poly(I:C) injection, confirming that inhibition of NK cell activation by Rhbdd3 contributes to the attenuation of acute liver inflammation by Rhbdd3. Interestingly, although greatly reduced, the level of IL-6 after NK cell depletion was still comparatively higher in Rhbdd3−/− mice than Rhbdd3+/+ mice, indicating that some unknown cells, other than NK cells, contribute to a small part of IL-6 in serum and have also been regulated by Rhbdd3. Notably, NK cell-depleted Rhbdd3−/− and Rhbdd3+/+ mice both had a 100% survival rate for at least 48 h after poly(I:C) injection (Fig. 6B), further confirming that Rhbdd3 inhibits poly(I:C)-induced mouse death through controlling NK cell function. Also, depletion of KCs with clodronate liposomes reduced poly(I:C)-induced ALT in Rhbdd3+/+ and Rhbdd3−/− mice to a similar level (Fig. 6C), further supporting our in vitro findings that Rhbdd3 negatively controls the interaction of NK cells and KCs.
Fig. 6.
Inhibition of NK cell function by Rhbdd3 contributes to attenuation of poly(I:C)-mediated acute liver inflammation. (A and B) Rhbdd3+/+ and Rhbdd3−/− mice were pretreated i.p. with anti-NK1.1 mAb (PK136) or isotype Ig (Isotype), followed by poly(I:C) and D-GalN injection 36 h later. After 24 h, serum ALT, AST, IFN-γ, and IL-6 levels were determined (six mice per group) (A). The survival rates were monitored for 48 h (10 mice per group). P < 0.01 by Wilcoxon test (B). (C) Rhbdd3+/+ and Rhbdd3−/− mice (six mice per group) were pretreated i.p. with clodronate liposomes or control liposomes, followed by poly(I:C) and D-GalN injection 36 h later. After 24 h, serum ALT levels were determined by ELISA. (D and E) Splenic NK cells from Rhbdd3+/+ or Rhbdd3−/− mice were i.v. transferred into WT mice, which were predepleted with NK cells by anti-NK1.1 mAb (4 × 106 cells per mouse) 36 h before. Mice were then i.p. injected with poly(I:C) and D-GalN 24 h later. After 24 h, serum ALT, AST, IFN-γ, and IL-6 levels were determined. The data shown are the means ± SD (A and C–E) from three independent experiments. *P < 0.05; **P < 0.01; NS, not significant.
Finally, we adoptively transferred Rhbdd3+/+ and Rhbdd3−/− NK cells into NK cell-depleted mice and then injected poly(I:C) and D-GalN. As expected, adoptive transfer of NK cells significantly restored the increased levels of ALT, AST, IFN-γ, and IL-6 in NK cell-depleted mice after poly(I:C) injection, and mice adoptively transferred with Rhbdd3−/− NK cells had higher levels of ALT, AST (Fig. 6D), IFN-γ, and IL-6 (Fig. 6E) than those with Rhbdd3+/+ NK cells. Together, the above data demonstrated that Rhbdd3 attenuates TLR3-triggered acute inflammation through controlling NK cell activation.
Discussion
Several negative regulators of NK cell activation have been identified, such as Ewing's sarcoma/friend leukemia virus integration 1 activated transcript 2 (27) and β-arrestin 2 (28). Our laboratory has demonstrated that the β2 integrin CD11b feedback inhibits NK cell function and, thus, attenuates TLR3-triggered acute liver inflammation (26). We have also found that micro-RNA-29 participates in the regulation of NK cell function through directly targeting Ifnγ mRNA (29). Here, we provide evidence that Rhbdd3 controls TLR3-triggered NK cell activation both in vitro and in vivo and, thus, identify a mechanism by which NK cell function is negatively regulated.
We found that poly(I:C) could only induce NK cell activation in the presence of cytokines such as IL-12/15 or accessory cells such as DCs and KCs, consistent with previous reports showing that NK cells could only be activated by poly(I:C) in the simultaneous presence of IL-12 or IL-8 (30). Moreover, Rhbdd3 inhibits TLR3-mediated NK cell activation only when DCs or KCs are presented. In fact, DC-mediated NK cell activation requires the formation of immune synapses, as well as soluble cytokines (24, 31). Thus, some ligands on DCs or KCs or receptors on NK cells might mediate the inhibitory effect of Rhbdd3 on TLR3-triggered NK cell activation. Interestingly, a poly(I:C)-inducible membrane protein referred to as IRF-3–dependent NK-activating molecule has been shown to mediate NK cell activation induced by DCs contact (32). It would be interesting to elucidate the role of IRF-3–dependent NK-activating molecule or other candidate molecules in the context of Rhbdd3-mediated inhibition of TLR3-triggered DC-NK cell interaction.
Notably, Rhbdd3 also regulates DC function to induce TLR3-triggered NK cell activation (Fig. 3 A and B). Indeed, Rhbdd3 is widely expressed in various organs and multiple types of immune cells. Although NK cells express higher levels of Rhbdd3 than DCs, Rhbdd3 is also up-regulated in DCs by TLR stimulation. Moreover, Rhbdd3−/− DCs expressed higher levels of CD86 and histocompatibility 2 class II antigen A beta 1 and produced more IL-12 and IL-15, but similar IFN-β, upon poly(I:C) stimulation than Rhbdd3+/+ DCs, indicating that Rhbdd3 also affects TLR3 signaling in DCs. Actually, older Rhbdd3−/− mice are prone to development of autoimmune disorders; it would, therefore, be intriguing to further investigate the cellular and molecular mechanisms for the control of autoimmunity by Rhbdd3.
MAPKs are the central transduction elements that mediate the signaling pathway triggered by various TLRs, including TLR3. Interestingly, whereas engagement of DAP12 by high-avidity ligands induces complete phosphorylation of immunoreceptor tyrosine-based activation motifs and recruitment of spleen tyrosine kinase, which synergizes with TLR-triggered MAPK and NF-κB pathway, low-avidity ligands induce a inhibitory signaling of DAP12 through activating the inhibitory phosphatase SH2-domain-containing protein tyrosine phosphatase 1, inhibiting the MAPK pathway (25). Thus, DAP12 may trigger a complex signaling network converging on MAPK and NF-κB activation. Here, we demonstrate that Rhbdd3 negatively regulates TLR3-trigered DAP12/MAPK signaling pathway without affecting NF-κB activation in NK cells and, thus, explain a mechanism for regulation of NK cell activation.
More NK cells accumulate in liver of Rhbdd3−/− mice upon poly(I:C) treatment, which might also contribute to the excessive liver injuries by poly(I:C). Chemokine receptors on NK cells such as chemokine receptor 2 (CCR2) (33) and CCR5 (34) have been indicated to regulate NK cell recruitment into liver. However, no difference in the expression of those molecules was found between Rhbdd3+/+ and Rhbdd3−/− mice (Fig. S8A). Besides, we found a remarkable increase in the level of IFN-γ–inducible protein 10 and stromal cell-derived factor-1α in poly(I:C)-treated mice, but their expression levels were not affected by Rhbdd3 deletion (Fig. S8B). Thus, some other molecules might have been involved in recruiting NK cells into liver.
Human RHBDD2/3 belongs to a subgroup of rhomboid proteins for which, to date, there has been no evidence that they are active proteases (17, 20). Rhbdd3 is the highly conserved homolog of RHBDD3 in mice. Thus, although Rhbdd3 is evolutionarily related with rhomboid proteins, the intermembrane protease function might not be so important for Rhbdd3 as for other rhomboid members such as RHBDD1. Notably, Rhbdd3 contains an ubiquitin-binding associated (UBA) domain, which regulates cellular processes through binding ubiquitin (35). Because Rhbdd3 can promote proteasomal degradation of DAP12, it is, thus, likely that Rhbdd3 might affect the degradation of DAP12 through ubiquitinization pathway via UBA domains, which awaits further investigation.
Here, we report the indispensable role of Rhbdd3 in inhibiting TLR3-triggered acute liver injury. In fact, previously published genome-profiling data also provide exciting information indicating the biological significance of Rhbdd3 in liver diseases. Based on three sets of genome-expression data of different hepatocellular carcinoma (HCC) mouse mutants (36–38) [Gene Expression Omnibus (GEO) database accession nos GDS2006, GDS1990, and GDS3087; www.ncbi.nlm.nih.gov/geo], Rhbdd3 is comparably reduced in HCC mutant mice than control mice. Chronic inflammation plays pivotal roles in promoting tumorigenesis. Proinflammatory cytokines such as IL-6 can enhance tumor progression, and anti-IL-6 drugs are being evaluated for cancer therapy (39). Thus, our study provide important clues to investigate the mechanisms underlying HCC pathogenesis. Altogether, our results shed a light on the mechanisms for the maintenance of immune homeostasis and provide a potential target for the treatment of liver inflammatory diseases.
Materials and Methods
Generation and Identification of Rhbdd3−/− Mice.
Details are described in SI Materials and Methods.
Materials.
Materials are described in SI Materials and Methods.
Cell Preparations.
Details are described in SI Materials and Methods.
Cocultures of NK Cells and DCs or Liver KCs.
Details are described in SI Materials and Methods.
Assay for NK Cell Cytotoxicity.
Details are described in SI Materials and Methods.
Confocal Analysis.
Details are described in SI Materials and Methods.
RNA Interference.
Details are described in SI Materials and Methods.
ELISA and Cytometric Bead Array Immunoassay.
Details are described in SI Materials and Methods.
Flow Cytometry.
Details are described in SI Materials and Methods.
Coimmunoprecipitation and Western Blotting.
Details are described in SI Materials and Methods.
Induction of Acute Liver Inflammation.
Details are described in SI Materials and Methods.
Statistical Analysis.
Student's t test was used to analyze statistical significance of differences for paired samples. Animal survival was analyzed using the Kaplan-Meir analysis and the survival rates were analyzed by the Wilcoxon's test. Statistical significance was determined as P < 0.05.
Supplementary Material
Acknowledgments
We thank Ms. Jinxia Jiang for excellent technical assistance. This work was supported by National Key Basic Research Program of China Grants 2013CB944903, 2012CB910202, and 2013CB530503; National Natural Science Foundation of China Grant 31070791; National High Biotechnology Development Program of China Grant 2012AA020808; and National 125 Key Project Grants 2012AA020901 and 2012ZX10002-014.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220466110/-/DCSupplemental.
References
- 1.Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9(5):481–485. doi: 10.1038/ni1583. [DOI] [PubMed] [Google Scholar]
- 2.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481(7381):394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang PA, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109(4):1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter AG, Smyth MJ. The role of NK cells in autoimmune disease. Autoimmunity. 2002;35(1):1–14. doi: 10.1080/08916930290005864. [DOI] [PubMed] [Google Scholar]
- 5.Mondelli MU, Varchetta S, Oliviero B. Natural killer cells in viral hepatitis: Facts and controversies. Eur J Clin Invest. 2010;40(9):851–863. doi: 10.1111/j.1365-2362.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsujimoto H, et al. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J Leukoc Biol. 2005;78(4):888–897. doi: 10.1189/jlb.0105051. [DOI] [PubMed] [Google Scholar]
- 8.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175(3):1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 9.Sivori S, Carlomagno S, Moretta L, Moretta A. Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells. Eur J Immunol. 2006;36(4):961–967. doi: 10.1002/eji.200535781. [DOI] [PubMed] [Google Scholar]
- 10.Hornung V, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168(9):4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 11.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Gately MK, et al. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 15.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11(10):658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou X, Zhou R, Wei H, Sun R, Tian Z. NKG2D-retinoic acid early inducible-1 recognition between natural killer cells and Kupffer cells in a novel murine natural killer cell-dependent fulminant hepatitis. Hepatology. 2009;49(3):940–949. doi: 10.1002/hep.22725. [DOI] [PubMed] [Google Scholar]
- 17.Lemberg MK, Freeman M. Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases. Genome Res. 2007;17(11):1634–1646. doi: 10.1101/gr.6425307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urban S. Rhomboid proteins: Conserved membrane proteases with divergent biological functions. Genes Dev. 2006;20(22):3054–3068. doi: 10.1101/gad.1488606. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. A novel member of the Rhomboid family, RHBDD1, regulates BIK-mediated apoptosis. Cell Mol Life Sci. 2008;65(23):3822–3829. doi: 10.1007/s00018-008-8452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abba MC, et al. Rhomboid domain containing 2 (RHBDD2): A novel cancer-related gene over-expressed in breast cancer. Biochim Biophys Acta. 2009;1792(10):988–997. doi: 10.1016/j.bbadis.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahar A, et al. Isolation and characterization of a novel pituitary tumor apoptosis gene. Mol Endocrinol. 2004;18(7):1827–1839. doi: 10.1210/me.2004-0087. [DOI] [PubMed] [Google Scholar]
- 22.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335(6065):225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIlwain DR, et al. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science. 2012;335(6065):229–232. doi: 10.1126/science.1214448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borg C, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104(10):3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 25.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7(2):155–161. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, et al. The beta2 integrin CD11b attenuates polyinosinic:polycytidylic acid-induced hepatitis by negatively regulating natural killer cell functions. Hepatology. 2009;50(5):1606–1616. doi: 10.1002/hep.23168. [DOI] [PubMed] [Google Scholar]
- 27.Roncagalli R, et al. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat Immunol. 2005;6(10):1002–1010. doi: 10.1038/ni1242. [DOI] [PubMed] [Google Scholar]
- 28.Yu MC, et al. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol. 2008;9(8):898–907. doi: 10.1038/ni.1635. [DOI] [PubMed] [Google Scholar]
- 29.Ma F, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol. 2011;12(9):861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 30.Sivori S, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: Induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101(27):10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretta A. Natural killer cells and dendritic cells: Rendezvous in abused tissues. Nat Rev Immunol. 2002;2(12):957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 32.Ebihara T, et al. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207(12):2675–2687. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J Immunol. 2005;174(3):1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]
- 34.Khan IA, et al. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2006;2(6):e49. doi: 10.1371/journal.ppat.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dikic I, Wakatsuki S, Walters KJ. Ubiquitin-binding domains - from structures to functions. Nat Rev Mol Cell Biol. 2009;10(10):659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheth SS, et al. Hepatocellular carcinoma in Txnip-deficient mice. Oncogene. 2006;25(25):3528–3536. doi: 10.1038/sj.onc.1209394. [DOI] [PubMed] [Google Scholar]
- 37.Katzenellenbogen M, et al. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66(8):4001–4010. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- 38.Khetchoumian K, et al. Loss of Trim24 (Tif1alpha) gene function confers oncogenic activity to retinoic acid receptor alpha. Nat Genet. 2007;39(12):1500–1506. doi: 10.1038/ng.2007.15. [DOI] [PubMed] [Google Scholar]
- 39.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.