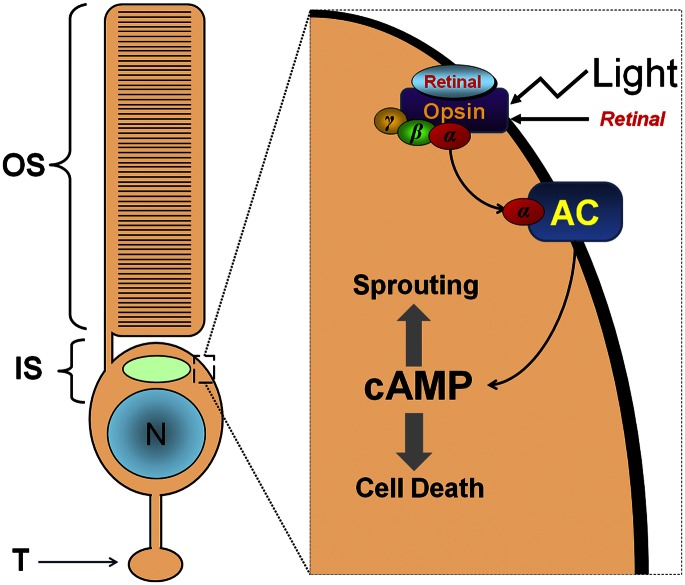
The article published by Cideciyan et al. (1) is an outstanding example of careful translational work. However, the concluding discussion warrants more thorough scrutiny. The authors proposed to increase the supply of chromophore (11-cis-retinal) to overcome the paradoxical improvement in visual function with continued photoreceptor degeneration seen in human patients with Leber congential amaurosis (LCA) treated by gene therapy. My own research on salamander retina warns against this approach. Specifically, we observed in isolated rod cells and in the intact neural retina that chromophore can cause rod cell degeneration and sprouting. Cell death (2) and sprouting (3) occurred if the receptor for chromophore, the G protein-linked protein opsin, mislocalized and appeared at noticeable levels in the plasma membranes of the inner segment (see Fig. 1 for mechanism). These results have recently been confirmed in zebra fish and the rd10 mouse. Therefore, the question is, when does opsin mislocalize in human LCA? And, does the introduction of chromophore into a retina with degeneration and abundant mislocalized opsin contribute to photoreceptor degeneration?
Fig. 1.
(Left) An intact rod photoreceptor cell. IS, inner segment; N, nucleus; OS, outer segment; T, terminal. (Right) A portion of the inner segment. In retinal disease, opsin, which normally is sequestered in the outer segment, appears on inner segment membranes. If bound by retinal and in the presence of light, opsin activates an abnormal signaling pathway involving adenylyl cyclase (AC) and leading to increased cAMP, neuritic sprouting, and cell death. Modified from (3), Copyright Association for Research in Vision and Ophthalmology.
Mislocalization of opsin to the membranes of the inner segment and synaptic terminal in rod photoreceptors is a feature of most known retinal degenerations regardless of the cause of degeneration [see discussion in Wang et al., (3)]. In human LCA, mislocalized opsin and rod cell sprouting have been reported in a 56-y-old patient (4). Because mislocalization occurs when rod cell degeneration occurs in other retinal diseases, mislocalization could be present much earlier in human LCA patients as soon as degeneration is present. In a canine model of LCA, a low level of mislocalized opsin was present in animals less than 1-y-old, preceding photoreceptor degeneration (5). Again, more mislocalization would be expected when degeneration is more pronounced. Thus, increasing the level of chromophore to retinas showing degeneration and opsin mislocalization, late in dogs but potentially much earlier in human retinas, will possibly serve to increase, not decrease, rod cell death.
Finally, it is interesting that in mouse models of LCA and perhaps human retina (4), cone cells show opsin mislocalization. In salamander, there was no evidence that chromophore binding to mislocalized cone opsin promotes cone cell death or sprouting. Indeed, the mechanism for cone sprouting appears to be different from that in rod cells (3). Thus, increased 11-cis-retinal will likely affect rod but not cone cell death directly.
I hope the authors of this thoughtful study on gene therapy in human and dog retina will heed this caution and reconsider their proposal to increase chromophore to treat LCA. Alternatively, blocking the activity of adenylyl cyclase may be a worthwhile experiment in animal models.
Footnotes
The author declares no conflict of interest.
References
- 1.Cideciyan AV, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA. 2013;110(6):E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfinito PD, Townes-Anderson E. Activation of mislocalized opsin kills rod cells: A novel mechanism for rod cell death in retinal disease. Proc Natl Acad Sci USA. 2002;99(8):5655–5660. doi: 10.1073/pnas.072557799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Zhang N, Beuve A, Townes-Anderson E. Mislocalized opsin and cAMP signaling: A mechanism for sprouting by rod cells in retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53(10):6355–6369. doi: 10.1167/iovs.12-10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonilha VL, et al. Histopathology and functional correlations in a patient with a mutation in RPE65, the gene for retinol isomerase. Invest Ophthalmol Vis Sci. 2011;52(11):8381–8392. doi: 10.1167/iovs.11-7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernández M, Pearce-Kelling SE, Rodriguez FD, Aguirre GD, Vecino E. Altered expression of retinal molecular markers in the canine RPE65 model of Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2010;51(12):6793–6802. doi: 10.1167/iovs.10-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]