Table 1.
H-bond distances for TyrD in QM/MM optimized geometries in the PSII protein environment (measured in angstroms)
| Redox/protonation state | OTyrD−Nε,His | OTyrD–H | H–Nε,His | OTyrD−OH2O | Nδ,His−NArg |
| Original (1.9-Å structure) | 2.74 | — | — | Distal, 4.30; proximal, 2.73 | 2.81 |
| TyrD-OH (Fig. 2, Left) | |||||
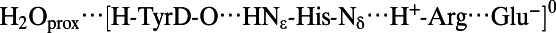
|
2.78 | 1.77 | 1.02 | 2.78 | 2.89 |
| TyrD-O• (Fig. 2, Right) | |||||
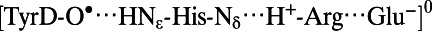
|
2.78 | 1.77 | 1.02 | 4.23 | 2.90 |
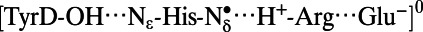
|
2.71 | 0.99 | 1.72 | 4.13 | 2.92 |
Note that the 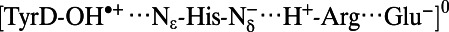 state was energetically very unstable and that only the
state was energetically very unstable and that only the 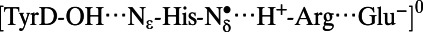 state was possible. Arg, D2-Arg294; Glu, CP47-Glu364; His, D2-His189; —, not applicable.
state was possible. Arg, D2-Arg294; Glu, CP47-Glu364; His, D2-His189; —, not applicable.
