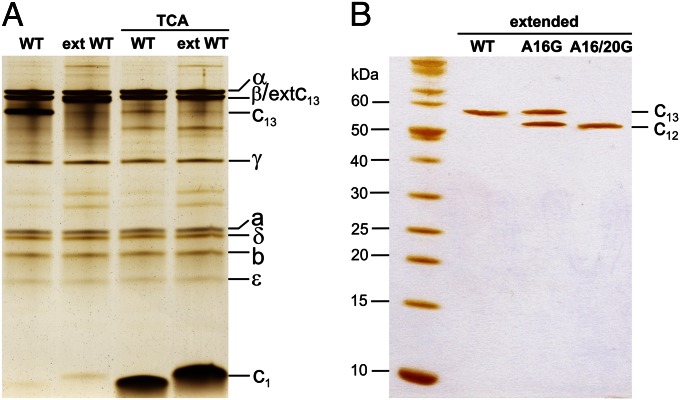
Fig. 3.
SDS/PAGE of purified B. pseudofirmus OF4 WT and alanine mutant ATP synthase and c-rings. (A) Isolated ATP synthases. F1Fo-ATP synthase with extended WT c-ring and WT c-ring before and after TCA treatment, which monomerizes the c-ring. The nonextended WT c13 and c1 (indicated) run faster than the extWT. (B) Isolated c-rings. The extWT c13 ring runs as a single band at ∼58 kDa; a molecular weight marker (in kDa) is on the left. The extA16G mutant shows two bands, one at the WT c13 level and the other at the c12 level. The double mutant extA16/20G runs as a single band, faster than extWT, at the c12 level. The samples were separated on 11% and 13.2% acrylamide gels in A and B, respectively, and stained with silver.