Abstract
Epidemiological studies from sub-Saharan Africa show that genital infection with Schistosoma haematobium may increase the risk for HIV infection in young women. Therefore, preventing schistosomiasis has the potential to reduce HIV transmission in sub-Saharan Africa. We developed a transmission model of female genital schistosomiasis and HIV infections that we fit to epidemiological data of HIV and female genital schistosomiasis prevalence and coinfection in rural Zimbabwe. We used the model to evaluate the cost-effectiveness of a multifaceted community-based intervention for preventing schistosomiasis and, consequently, HIV infections in rural Zimbabwe, from the perspective of a health payer. The community-based intervention combined provision of clean water, sanitation, and health education (WSH) with administration of praziquantel to school-aged children. Considering variation in efficacy between 10% and 70% of WSH for reducing S. haematobium transmission, our model predicted that community-based intervention is likely to be cost-effective in Zimbabwe at an aggregated WSH cost corresponding to US $725–$1,000 per individual over a 20-y intervention period. These costs compare favorably with empirical measures of WSH provision in developing countries, indicating that integrated community-based intervention for reducing the transmission of S. haematobium is an economically attractive strategy for reducing schistosomiasis and HIV transmission in sub-Saharan Africa that would have a powerful impact on averting infections and saving lives.
Keywords: cost-effectiveness analysis, mathematical modeling, schistosomiasis control
Schistosomiasis, transmitted by the water-borne parasite Schistosoma haematobium (1, 2), is highly prevalent in sub-Saharan Africa, where it is primarily acquired during childhood (3, 4). S. haematobium migrates largely to the bladder but is also found in adjacent areas, such the genital tract, causing ulcerative lesions around the vagina and cervix and resulting in a condition known clinically as female genital schistosomiasis (FGS) (2). Several cross-sectional epidemiological studies have reported that in regions most heavily affected by the HIV/AIDS pandemic, women with FGS have a three- to fourfold increased odds of having HIV compared with women without FGS (4–6). The FGS-mediated breach in the epithelial barriers of the cervix, as well as inflammation of the genital mucosal tissues, appears to increase HIV susceptibility (4, 7). The strong statistical association between FGS and HIV, the biological plausibility of the association, and the observation that schistosomal genital lesions are common in FGS-infected women before puberty (3, 5, 6) together provide convincing evidence that FGS is a significant risk factor for HIV infection in sub-Saharan Africa.
The World Health Organization (WHO) recommends preventive chemotherapy as a global strategy for the control of schistosomiasis morbidity (8). The emphasis of this strategy is routine administration of praziquantel to school-aged children, the age class at greatest risk for highest levels of schistosomiasis infection because their immune system is not as effective in mounting a response to the infection (8, 9). Praziquantel is a potent anthelminthic chemotherapy that can reduce schistosomal morbidity, such as FGS and its clinical manifestations associated with exacerbated HIV susceptibility (10, 11). However, once schistosomiasis infection has been established for a long time, its symptoms can persist even after treatment (11, 12). Additionally, morbidity arising from posttransmission schistosomiasis often persists as clinical symptoms even for many years after transmission has been interrupted (13). Praziquantel must be repeatedly administered to prevent schistosomiasis reinfection, a common problem in areas endemic for schistosomiasis (14, 15). Therefore, complementary community control measures are simultaneously needed to curtail schistosomiasis sustainably (15, 16). Specifically, reducing the contact rate of a community with schistosome-infested water may be achieved through provision of clean water, sanitation, and health education (WSH) (14, 16–18).
We conducted a cost-effectiveness analysis of a community-based intervention for schistosomiasis control, combining provision of WSH and annual praziquantel administration to school-aged children, taking into account the impact on both S. haematobium and HIV. Focusing on the perspective of health payers, such as national governments or international donors, which are the major providers for schistosomiasis control and HIV antiretroviral therapy in sub-Saharan Africa (19–21), we constructed a transmission model of the joint dynamics of HIV and FGS, parameterized with epidemiological data from a cross-sectional study of rural Zimbabwean women (2, 6). We evaluated the costs and disability-adjusted life years (DALYs) averted, from which we calculated the WSH cost thresholds at which the community-based intervention is cost-effective and very cost-effective, defined as a cost-effectiveness ratio less than threefold the per capita gross domestic product (GDP) and less than onefold the per capita GDP, respectively. We show that this combined community-based intervention for schistosomiasis control may be an effective approach to prevent both HIV and schistosomiasis infections in S. haematobium-endemic areas.
Results
We fit our transmission model of HIV and FGS dynamics to epidemiological HIV/FGS data from rural Zimbabwe (2, 6) using a Bayesian Markov chain Monte Carlo (MCMC) method. The MCMC method draws values of parameters from prior distributions, based on empirical estimates available in the literature, to derive posterior distributions for these parameters (Table 1). Probabilistically capturing prior knowledge provides increased precision and accuracy not only for values of these previously described parameters but for determining unknown or uncertain parameters. Thus, the MCMC method facilitates a realistic calibration of the transmission model to HIV and FGS prevalences.
Table 1.
Estimates of the parameters used in our dynamic HIV-FGS model
| Variable | Meaning | Mean (95% CI) | BGR diagnostic upper CI limit* |
 |
Probability of acquiring FGS, given childhood infection | 0.457 (0.335–0.708) | 1.193 |
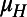 |
Duration of HIV/AIDS infection | 8.533 (7.725–9.106) | 1.077 |
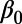 |
Intrinsic HIV transmission rate | 0.317 (0.285–0.355) | 1.079 |
 |
Relative increase HIV transmission from men | 1.112 (1.004–1.388) | 1.025 |
 |
Reduction rate of HIV transmission | 5.096 (3.316–7.093) | 1.131 |
 |
Scale of influence of deaths on HIV transmission | 1.413 (1.172–1.648) | 1.105 |
 |
Probability of acquiring FGS as a result of adulthood infection | 0.017 (0.006–0.027) | 1.183 |
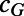 |
Enhance HIV transmission in FGS-infected women | 1.758 (1.142–2.404) | 1.130 |
These parameter estimates produced the best fit of our dynamic model to epidemiological data for HIV and FGS prevalence and coinfection among rural Zimbabwean women (2, 6). The dynamic model was fit to these data using a Bayesian MCMC method to allow calculation of distributions for possible values for each of these parameters. The means of these distributions and their associated 95% credible intervals (CIs) are shown.
Brooks–Gelman–Rubin (BGR) method monitors convergence of iterative simulations. If the upper limit of the credible interval of the BGR diagnostic statistic for a given parameter is <1.2, that parameter is considered to have converged to a robust solution (35).
The best estimates and 95% credible interval of the model predictions were compared with the empirical data from a cross-sectional epidemiological study among rural Zimbabwean women (2, 6). The FGS prevalence in this model was estimated to be 46.5% (range: 40.8–51.0%) compared with the empirical prevalence of 46.1% (range: 41.8–50.5%) in Zimbabwe. Likewise, the HIV prevalence was estimated to be 29% (range: 26.1–32.0%) compared with the empirical prevalence of 28.1% (range: 24.0–32.5%). Furthermore, the odds ratio of the association between FGS and HIV was estimated to be 2.0 (range: 1.1–3.4) compared with the empirical odds ratio of 2.1 (range: 1.2–3.5) in rural Zimbabwe.
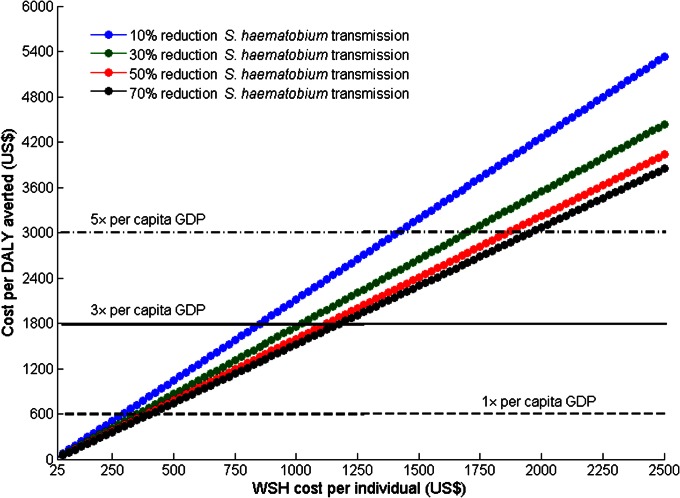
We used the mean values of the estimated and empirical epidemiological parameters of the transmission model input (Table 1 and Supporting Information) and cost data (Table 2) to conduct our base case analysis. Using different costs of WSH, and considering a wide range of potential WSH efficacy for reducing S. haematobium transmission from contaminated water, we evaluated the cost-effectiveness of the community-based intervention (Fig. 1). The WSH costs were given as aggregated costs of preparation, installation, maintenance, and operational costs of a water supply system and sanitation facilities over the duration of the intervention period (here, evaluated over 20 y). We showed that even for a WSH efficacy as low as 30%, the community-based intervention was cost-effective for reducing S. haematobium and HIV infections in Zimbabwe at a threshold WSH cost of US $875 per individual over a 20-y period (Fig. 1). The intervention was very cost-effective when the WSH cost per individual was lower than US $300 over the same period (Fig. 1).
Table 2.
Baseline estimates and distributions of praziquantel costs and efficacies as well as antiretroviral therapy coverage
| Variable | Baseline value, US $ | Distribution | Ref(s). |
| Cost per praziquantel tablet (600 mg) | 0.08 | NA | 30 |
| Cost of delivery of praziquantel per individual | 0.21 | Min = 0.06, Max = 0.81 (uniform) | 42 |
| Efficacy of MDA praziquantel (schistosomiasis cure rate) | 0.80 | Min = 0.57, Max = 0.93 (uniform) | 30 |
| Efficacy of MDA praziquantel (reduction of the risk for acquiring FGS) | 0.5 | Min = 0.4, Max = 0.7 (uniform) | 11 |
| ART coverage (proportion HIV patients receiving ART) | 0.34 | Mean = 0.34, SD = 0.02 (normal) | 33 |
| Zimbabwe non-HIV/AIDS health expenditure (cost per person per annum) | 26 | Mean = 26, SD = 4.8 (gamma) | 21, 43 |
| Cost lifetime ART (ARV first line, ARV second line, ARV monitoring) | 3,000 | NA | 55 |
| Other lifetime cost of HIV treatment (prophylaxis and treatment of opportunistic infections, diagnostic and routine testing, palliative care) | 695 | NA | 55 |
ART, antiretroviral therapy; ARV, antiretroviral; MDA, mass drug administration; Max, maximum; Min, minimum; NA, not applicable.
Fig. 1.
Cost-effectiveness of community-based intervention for the base case analysis. The cost per DALY averted was computed for different values of the cost of WSH per individual and for different levels of WSH efficacy in reducing S. haematobium transmission.
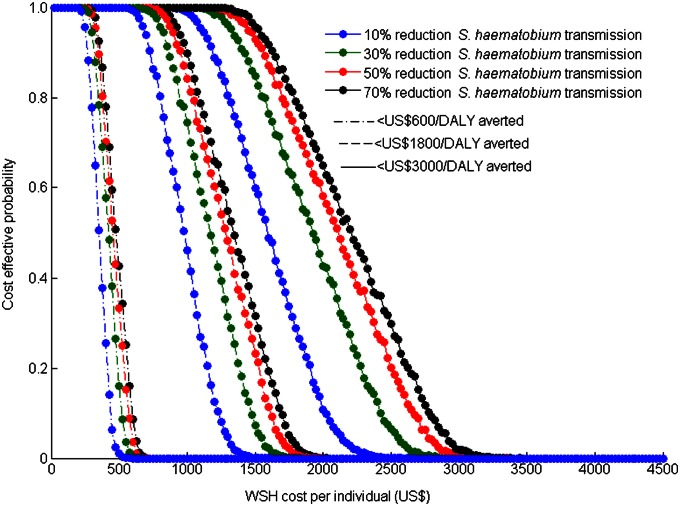
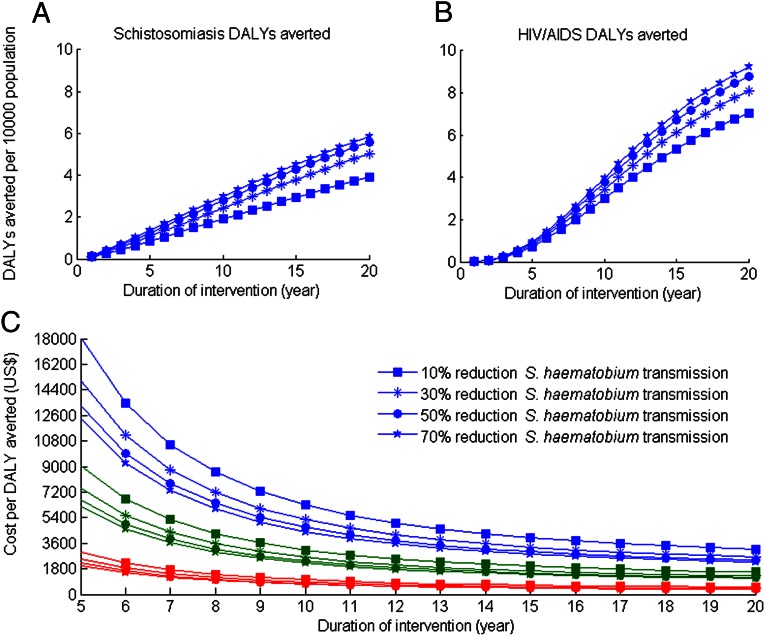
Our probabilistic sensitivity analysis showed that the cost-effectiveness of the community-based intervention was more sensitive to the cost of providing WSH than to the efficacy of WSH over the broad range considered (Fig. 2). Specifically, we found that for WSH efficacy ranging from 10–70%, the cost of WSH per individual should range from US $725–$1,000 over a 20-y period to achieve a 90% probability of being cost-effective. To achieve a 90% probability of being very cost-effective, the cost of WSH per individual should range from US $275–$350 over a 20-y period, again depending on the WSH efficacy (Fig. 2). Additionally, the cost of WSH per individual should range from US $1,175–$1,625 to achieve a 90% probability of being cost-effective for a willingness-to-pay threshold of fivefold the per capita GDP (Fig. 2). We also found that the cost-effectiveness of the community-based intervention may vary significantly with its duration. As the duration of the intervention increases, the community-based intervention may shift from not being cost-effective to being very cost-effective (Fig. 3), primarily as a result of the time required for the full effectiveness of the intervention to be realized. For example at a WSH cost of US $250 per individual, the community-based intervention was not cost-effective for a 5-y intervention period but was cost-effective for a 7- to 12-y intervention period, and very cost-effective for an intervention period of more than 14 y (Fig. 3).
Fig. 2.
Effect of the cost of WSH per individual and the efficacy of WSH in reducing S. haematobium transmission on the probability that the community-based intervention will be cost-effective (dashed lines), will be very cost-effective (dashed-dotted lines), and will meet fivefold the GDP threshold criteria (solid lines).
Fig. 3.
Effectiveness and cost-effectiveness of the community-based intervention as a function of the duration of the intervention. Annual discounted number of DALYs saved from S. haematobium (A), annual discounted number of DALYs saved from HIV/AIDS (B), and the cost-effectiveness ratio (C) were computed when costs of providing WSH per individual were US $250 (red lines), US $750 (green lines), and US $1,500 (blue lines), respectively.
Discussion
We evaluated the cost-effectiveness of a community-based intervention for averting infections of both S. haematobium and HIV. The intervention integrated the provision of WSH for the entire community with praziquantel treatment of school-aged children. Our results indicate that this integrated community-based approach toward schistosomiasis control could effectively reduce the health and economic burden associated with S. haematobium and HIV infections in sub-Saharan Africa. The cost-effectiveness of the community-based intervention was found to vary primarily with the cost of WSH, and secondarily with the efficacy of WSH for reducing S. haematobium transmission, as well as with the duration of the intervention. Our model predicted that for a wide range of WSH efficacies ranging from 10–70% in terms of reducing S. haematobium transmission, the community-based intervention is likely to be cost-effective in Zimbabwe at an aggregated WSH cost of US $725–$1,000 per individual over a 20-y intervention period. These threshold costs compare favorably with empirical estimates of expenditure of US $200–$1,020 for providing sustainable basic water and sanitation services in developing countries (22).
Current control of schistosomiasis focuses on reducing schistosomiasis-induced morbidity by implementing large-scale preventive chemotherapy programs through administration of praziquantel to school-aged children (23). Although praziquantel has an appreciable immediate reduction on schistosomiasis morbidity (24), the high frequency of posttreatment reinfection, especially in endemic areas (14, 15), means that praziquantel treatment alone may have a limited impact on the long-term control of schistosomiasis (16, 25). To ensure sustainability of schistosomiasis control, it is essential to integrate praziquantel administration with provision of WSH, which is necessary for reducing schistosomiasis transmission (16, 26). Cooperative ventures between pharmacological interventions and engineering-oriented programs for the provision of clean water and sanitation would be ideal for promoting sustainable approaches to schistosomiasis control. Health education is a fundamental component of this integrated approach, given that there must be a significant societal change in water use and sanitation to leverage the provision of clean water supply and sanitation to curtail schistosomiasis transmission (27, 28).
Our evaluations of the cost-effectiveness of the community-based intervention are conservative, because clean water supply and sanitation would not only contribute to the reduction in schistosomiasis transmission but would prevent a conglomerate of diseases, such as diarrheal diseases, soil-transmitted helminths, and bacterial infections (29). Thus, consideration of these additional public health benefits would further enhance the cost-effectiveness of the community-based intervention.
FGS prevalence among adult women is associated with S. haematobium prevalence rather than with infection intensity (10). However, it has not been evaluated clinically whether FGS prevalence may be associated with infection intensity in children. Epidemiological data on the association between infection intensity and FGS prevalence would be needed to extend our analysis to determine the impact of worm burden heterogeneity on community-based intervention effectiveness. Given that praziquantel efficacy may decrease with worm burden (30, 31), mass praziquantel administration may be less effective for reducing FGS for individuals with a high worm burden than for those with a low worm burden, as well as generating variation in effectiveness among communities depending on respective distributions of worm burdens.
Genital schistosomiasis may interact with other risk factors, such as the presence of other genital ulcerative diseases, age at sexual debut, and number of sexual partners, in increasing the risk for HIV infection. Nevertheless, the biological plausibility of elevated susceptibility to HIV infection arising from the FGS-mediated genital ulcerative lesions and immunomodulatory effects (4) suggests that FGS prevention could significantly reduce HIV transmission in communities endemic for S. haematobium. Consequently, a WHO working group on urogenital schistosomiasis and HIV transmission has proposed a prospective study to evaluate the effect of praziquantel treatment on HIV incidence as the next step toward developing a new protocol to treat schistosomiasis for HIV prevention (3). Future analysis could be extended by investigating the effectiveness of schistosomiasis control on HIV transmission in the context of other HIV transmission cofactors and expansion of other HIV control strategies, such as antiretroviral therapy, male circumcision, and HIV counseling and testing (32, 33).
Our study evaluates the cost-effectiveness of community-based intervention to reduce both schistosomiasis and HIV in sub-Saharan Africa. We demonstrate that integrating provision of WSH with praziquantel administration may generate indirect benefits for HIV prevention that extend beyond the benefits of reducing the schistosomiasis burden itself, yielding substantial economic and public health benefits.
Methods
We developed a compartmental model for the coinfection dynamics of genital S. haematobium and HIV. We used a Bayesian MCMC (34, 35) approach to fit the model to epidemiological data on the prevalence of FGS, HIV, and coinfection of rural Zimbabwean women (2, 6). The MCMC approach allowed us to estimate uncertain epidemiological parameters by combining prior information about these parameters from epidemiological studies, empirical HIV and FGS data, and dynamic model prevalence predictions (details on model and parameterization are provided in SI Text, Web Appendix).
Community-Based Intervention.
We considered a community-based intervention for schistosomiasis control, including annual praziquantel administration to school-aged children, provision of safe water through improved water supply (e.g., piped supplies), provision of sanitation through the construction of ventilated improved pit-type latrines, and health education campaigns. Praziquantel is both a highly effective antischistosomal therapy and a prophylactic agent against schistosomal morbidity (19) with no serious or long-lasting adverse effects (36, 37).
We modeled annual praziquantel administration targeted at school-aged children based on the WHO recommendation for schistosomiasis control in endemic areas (8). We assumed that girls who have been treated with praziquantel were less likely to develop FGS than those who had not been treated (38, 39). We differentiated between the efficacy of the community-based intervention for reducing S. haematobium morbidity through praziquantel treatment and its efficacy for reducing S. haematobium transmission through the collective effects of WSH based on the risk for S. haematobium reinfection (Table 2).
We parameterized our model with data from the Mount Darwin district, which is a rural district of the Mashonaland Central province of Zimbabwe, where the Zimbabwean cross-sectional epidemiological study on the association between FGS and HIV was conducted (2, 6). We assumed that the population of the Mount Darwin district was 150,000 in 2000 (40, 41).
Costs.
Because national health care systems and international donors are the primary providers of treatment costs for HIV and schistosomiasis control in Africa (19–21), we calculated the cost-effectiveness from a health care system perspective. Thus, only direct medical costs to the health provider were considered, including the costs of praziquantel administration, lifetime treatment costs of an HIV infection, and health provider expenditures unrelated to HIV/AIDS, as well as the costs of providing WSH.
The costs of praziquantel administration included the cost of the drug [US $0.08 (30)], delivery, training, social mobilization, capital equipment, and administrative costs [US $0.21 (42)]. We assumed that treatment was administered according to the WHO recommended dose for praziquantel (2.5 tablets of 600 mg per child per year) (8). Medical costs for HIV treatment and care included provider-initiated testing (both diagnostic and monitoring), treatment and prophylaxis for opportunistic infections, antiretroviral therapy, and palliative care. Other than HIV-related spending, the Zimbabwean government expenditure on health was estimated to be US $26 per capita annually (21, 43). We aggregated the cost of providing WSH over the length of the intervention period, including preparation, installation, maintenance, and operational costs. Costs were discounted at an annual rate of 3%, according to WHO recommendations (44).
Effectiveness.
We quantified the effectiveness of the community-based intervention on schistosomiasis and HIV in terms of DALYs, which is a common measure for health burden resulting from mortality and disability (45). The average period of HIV infection ( ) was estimated by fitting the model to epidemiological data, as indicated above. We assumed that the duration of HIV infection includes
) was estimated by fitting the model to epidemiological data, as indicated above. We assumed that the duration of HIV infection includes  y of limited morbidity with a disability weighting of 0.135 (range: 0.123–0.136) and 1 y of severe disease with a disability weighting of 0.505 (46). We also assumed that HIV-infected individuals who receive antiretroviral therapy have a disability weighting of 0.167 (range: 0.145–0.469) (46, 47). The antiretroviral therapy coverage in sub-Saharan Africa was assumed to be 37% (range: 34–40%) (33). The average age at HIV/AIDS acquisition was assumed to be 25 y (48), and the life expectancy at the age of 25 y was estimated to be 40 y (49). The disability weight associated with S. haematobium infection was assumed to be 0.05 (range: 0.005–0.15) (30, 46). DALYs were computed with a 3% annual discount rate and without age weighting (44, 50).
y of limited morbidity with a disability weighting of 0.135 (range: 0.123–0.136) and 1 y of severe disease with a disability weighting of 0.505 (46). We also assumed that HIV-infected individuals who receive antiretroviral therapy have a disability weighting of 0.167 (range: 0.145–0.469) (46, 47). The antiretroviral therapy coverage in sub-Saharan Africa was assumed to be 37% (range: 34–40%) (33). The average age at HIV/AIDS acquisition was assumed to be 25 y (48), and the life expectancy at the age of 25 y was estimated to be 40 y (49). The disability weight associated with S. haematobium infection was assumed to be 0.05 (range: 0.005–0.15) (30, 46). DALYs were computed with a 3% annual discount rate and without age weighting (44, 50).
The annual number of DALYs averted was calculated by multiplying the annual number of infections averted for HIV and S. haematobium by the total DALYs averted per case due to averted disability and premature death (50). These estimates of the disability weights associated with S. haematobium are conservative because they do not include morbidity associated with FGS specifically (10, 30).
Cost-Effectiveness Framework.
We calculated the cost-effectiveness of community-based intervention for schistosomiasis control, with the status quo of no mass administration of praziquantel, no health education campaign, and no sanitation campaign against schistosomiasis, as is currently the case in many rural Zimbabwean districts. We measured the effectiveness of the intervention strategy in terms of DALYs for schistosomiasis and HIV averted over a baseline intervention period of 20 y. The baseline intervention period was chosen to be equal to the average lifespan of latrines and standpipes (51). We estimated the Zimbabwean per capita GDP at US $600 (52, 53) and the commonly used willingness-to-pay values of threefold the per capita GDP (US $1,800) for the cost-effective threshold and of the per capita GDP (US $600) for the very cost-effective threshold, in accordance with the WHO criteria (54). We also explored an alternative threshold of fivefold the per capita GDP (US $3,000).
We conducted the cost-effectiveness analysis from the perspective of health payers, such as the national government or international donors, which are the major providers for schistosomiasis control and HIV antiretroviral therapy in sub-Saharan Africa (19–21).
Probabilistic Sensitivity Analyses.
We conducted probabilistic sensitivity analyses to assess the impact of the efficacy of WSH for reducing schistosomiasis transmission on the cost-effectiveness ratio of the integrated community-based intervention. For a given level of WSH efficacy, we randomly sampled the posterior distributions of the epidemiological parameters (Table 1) and probability distributions of the cost and efficacy of praziquantel administration, as well as antiretroviral therapy coverage and non-HIV/AIDS health expenditure obtained from epidemiological studies (Table 2), to generate 10,000 independent model outcomes. The outcomes were used both to evaluate the impact of parameter uncertainty on the cost-effectiveness ratios and to estimate the probability of being cost-effective and very cost-effective for different efficacies of WSH.
Supplementary Material
Acknowledgments
This study was funded by the National Institute of General Medical Sciences (Models of Infectious Disease Agent Study Grant U01GM087719).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221396110/-/DCSupplemental.
References
- 1.van der Werf MJ, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86(2-3):125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 2.Kjetland EF, et al. Simple clinical manifestations of genital Schistosoma haematobium infection in rural Zimbabwean women. Am J Trop Med Hyg. 2005;72(3):311–319. [PubMed] [Google Scholar]
- 3.WHO 2010. Report of an informal working group meeting on urogenital schistosomiasis and HIV transmission (WHO, Geneva). Available at . http://whqlibdoc.who.int/hq/2010/WHO_HTM_NTD_PCT_2010.5_eng.pdf. Accessed November 21, 2012.
- 4.Mbabazi PS, et al. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl Trop Dis. 2011;5(12):e1396. doi: 10.1371/journal.pntd.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downs JA, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg. 2011;84(3):364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjetland EF, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20(4):593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 7.Jourdan PM, Holmen SD, Gundersen SG, Roald B, Kjetland EF. HIV target cells in Schistosoma haematobium-infected female genital mucosa. Am J Trop Med Hyg. 2011;85(6):1060–1064. doi: 10.4269/ajtmh.2011.11-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO 2011. Helminth Control in School-Age Children. A Guide for Managers of Control Programmes. Preventive Chemotherapy and Transmission Control (PCT), Department of Control of Neglected Tropical Diseases (NTD) (WHO, Geneva). Available at http://whqlibdoc.who.int/publications/2011/9789241548267_eng.pdf. Accessed August 13, 2012.
- 9.Woolhouse ME, Taylor P, Matanhire D, Chandiwana SK. Acquired immunity and epidemiology of Schistosoma haematobium. Nature. 1991;351(6329):757–759. doi: 10.1038/351757a0. [DOI] [PubMed] [Google Scholar]
- 10.Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. 2012;28(2):58–65. doi: 10.1016/j.pt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Kjetland EF, et al. Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. Am J Trop Med Hyg. 2008;79(1):79–83. [PubMed] [Google Scholar]
- 12.Poggensee G, et al. Female genital schistosomiasis of the lower genital tract: Prevalence and disease-associated morbidity in northern Tanzania. J Infect Dis. 2000;181(3):1210–1213. doi: 10.1086/315345. [DOI] [PubMed] [Google Scholar]
- 13.Giboda M, Bergquist N. Post-transmission schistosomiasis: A new agenda. Acta Tropica. 2000;77:3–7. doi: 10.1016/s0001-706x(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 14.Singer BH, de Castro MC. Bridges to sustainable tropical health. Proc Natl Acad Sci USA. 2007;104(41):16038–16043. doi: 10.1073/pnas.0700900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utzinger J, et al. Schistosomiasis and neglected tropical diseases: Towards integrated and sustainable control and a word of caution. Parasitology. 2009;136(13):1859–1874. doi: 10.1017/S0031182009991600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiegel JM, et al. Which new approaches to tackling neglected tropical diseases show promise? PLoS Med. 2010;7(5):e1000255. doi: 10.1371/journal.pmed.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Utzinger J, Bergquist R, Shu-Hua X, Singer BH, Tanner M. Sustainable schistosomiasis control—The way forward. Lancet. 2003;362(9399):1932–1934. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- 18.Asaolu SO, Ofoezie IE. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003;86(2-3):283–294. doi: 10.1016/s0001-706x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 19.Hotez PJ, Fenwick A, Kjetland EF. Africa’s 32 cents solution for HIV/AIDS. PLoS Negl Trop Dis. 2009;3(5):e430. doi: 10.1371/journal.pntd.0000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abt Associates, Inc. 2005. Zimbabwe HIV and AIDS Subaccounts 2005 (Abt Associates, Bethesda). Available at www.equinetafrica.org/bibl/docs/ABTaids020309.pdf. Accessed November 16, 2012.
- 21.Amico P, Aran C, Avila C. HIV spending as a share of total health expenditure: An analysis of regional variation in a multi-country study. PLoS ONE. 2010;5(9):e12997. doi: 10.1371/journal.pone.0012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IRC International Water and Sanitation Centre 2012. Providing a basic level of water and sanitation services that last: Cost benchmarks. Available at www.washcost.info/page/2386. Accessed November 21, 2012.
- 23.Fenwick A, et al. The Schistosomiasis Control Initiative (SCI): Rationale, development and implementation from 2002-2008. Parasitology. 2009;136(13):1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- 24.Miguel E, Kremer M. Worms: Identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72:159–217. [Google Scholar]
- 25.Clements ACA, et al. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984-1989 and 2004-2006. PLoS Negl Trop Dis. 2009;3(5):e431. doi: 10.1371/journal.pntd.0000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King CH. Parasites and poverty: The case of schistosomiasis. Acta Trop. 2010;113(2):95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruun B, Aagaard-Hansen J. The Social Context of Schistosomiasis and Its Control. Geneva: WHO; 2008. [Google Scholar]
- 28.WHO . Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases. A Roadmap for Implementation. Executive Summary. Geneva: WHO; 2012. Available at http://whqlibdoc.who.int/hq/2012/WHO_HTM_NTD_2012.1_eng.pdf. Accessed November 21, 2012. [Google Scholar]
- 29.Bartram J, Cairncross S. Hygiene, sanitation, and water: Forgotten foundations of health. PLoS Med. 2010;7(11):e1000367. doi: 10.1371/journal.pmed.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King CH, et al. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: A systematic review. PLoS Negl Trop Dis. 2011;5(9):e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson RM, May RM, Anderson B. Infectious Diseases of Humans: Dynamics and Control (Oxford Science Publications) New York: Oxford Univ Press; 1992. Accessed January 8, 2013. [Google Scholar]
- 32.Bärnighausen T, Bloom DE, Humair S. Economics of antiretroviral treatment vs. circumcision for HIV prevention. Proc Natl Acad Sci USA. 2012;109(52):21271–21276. doi: 10.1073/pnas.1209017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO 2010. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector: Progress report 2010 (WHO, Geneva). Available at www.who.int/hiv/pub/2010progressreport/en/. Accessed November 21, 2012.
- 34.Kloek T, van Dijk HK. Bayesian estimates of equation system parameters: An spplication of integration by Monte Carlo. Econometrica. 1978;46:1–19. [Google Scholar]
- 35.Brooks A, Gelman S. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1999;7(4):434–455. [Google Scholar]
- 36.Kabatereine NB, et al. Efficacy and side effects of praziquantel treatment in a highly endemic Schistosoma mansoni focus at Lake Albert, Uganda. Trans R Soc Trop Med Hyg. 2003;97(5):599–603. doi: 10.1016/s0035-9203(03)80044-5. [DOI] [PubMed] [Google Scholar]
- 37.Midzi N, et al. Efficacy and side effects of praziquantel treatment against Schistosoma haematobium infection among primary school children in Zimbabwe. Trans R Soc Trop Med Hyg. 2008;102(8):759–766. doi: 10.1016/j.trstmh.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Richter J, et al. Reversibility of lower reproductive tract abnormalities in women with Schistosoma haematobium infection after treatment with praziquantel—An interim report. Acta Trop. 1996;62(4):289–301. doi: 10.1016/s0001-706x(96)00030-7. [DOI] [PubMed] [Google Scholar]
- 39.Kjetland EF, et al. Prevention of gynecologic contact bleeding and genital sandy patches by childhood anti-schistosomal treatment. Am J Trop Med Hyg. 2008;79(1):79–83. [PubMed] [Google Scholar]
- 40.CSOMI 2000. Zimbabwe Demographic and Health Survey (ICF International, Calverton, MD). Available at www.measuredhs.cam/pubs/pdf/FR116/FR116.pdf. Accessed November 16, 2012.
- 41.UN . World Urbanization Prospects: The 2007 Revision. New York: United Nations; 2008. Available at www.un.org/esa/population/publications/wup2007/. Accessed November 21, 2012. [Google Scholar]
- 42.Goldman AS, et al. National mass drug administration costs for lymphatic filariasis elimination. PLoS Negl Trop Dis. 2007;1(1):e67. doi: 10.1371/journal.pntd.0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO . WHO Global Health Expenditure Database. Geneva: WHO; 2011. Available at www.who.int/nha/database. Accessed November 8, 2011. [Google Scholar]
- 44.Gold M. Cost-Effectiveness in Health and Medicine. New York: Oxford Univ Press; 1996. Available at www.amazon.com/Cost-Effectiveness-Health-Medicine-Marthe-Gold/dp/0195108248. Accessed November 12, 2012. [Google Scholar]
- 45.Murray CJ. Quantifying the burden of disease: The technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72(3):429–445. [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez AD, Mathers C, Ezzati M, Jamison D, Murray C. Global Burden Of Disease And Risk Factors. New York: World Bank Publications; 2006. Available at http://books.google.com/books?hl=en&lr=&id=F8Abr-ofOwIC&pgis=1. Accessed November 21, 2012. [PubMed] [Google Scholar]
- 47.Hogan DR, Baltussen R, Hayashi C, Lauer JA, Salomon JA. Cost effectiveness analysis of strategies to combat HIV/AIDS in developing countries. BMJ. 2005;331(7530):1431–1437. doi: 10.1136/bmj.38643.368692.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munjoma MW, et al. The incidence of HIV among women recruited during late pregnancy and followed up for six years after childbirth in Zimbabwe. BMC Public Health. 2010;10:668. doi: 10.1186/1471-2458-10-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO . Global Health Observatory Database. Geneva: WHO; 2010. Life-Tables: Zimbabwe. Available at http://apps.who.int/ghodata. Accessed June 3, 2011. [Google Scholar]
- 50.Hollinghurst S, Bevan G, Bowie C. Estimating the “avoidable” burden of disease by Disability Adjusted Life Years (DALYs) Health Care Manage Sci. 2000;3(1):9–21. doi: 10.1023/a:1019016702081. [DOI] [PubMed] [Google Scholar]
- 51.WHO . Evaluation of the Costs and Benefits of Water and Sanitation Improvements at the Global Level. Geneva: WHO; 2004. Available at www.who.int/water_sanitation_health/wsh0404/en/. Accessed November 16, 2012. [Google Scholar]
- 52.World Bank . GDP per Capita. New York: World Bank Publications; 2011. Available at http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed November 8, 2011. [Google Scholar]
- 53.IMF 2012. World Economic Outlook Database List (International Monetary Fund, Washington). Available at www.imf.org/external/ns/cs.aspx?id=28. Accessed November 16, 2012.
- 54.WHO Commission on Macroeconomics and Health . Macroeconomics and Health: Investing in Health for Economic Development. Geneva: WHO; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.