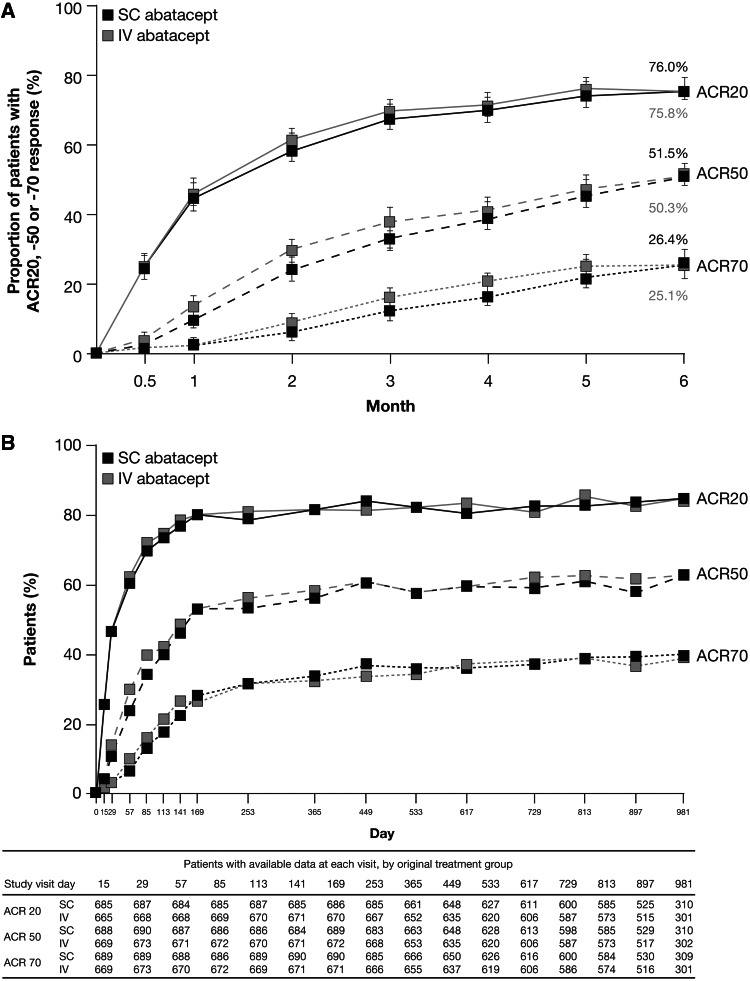
Fig. 1.
Proportion of s.c. or i.v. abatacept-treated patients in the ACQUIRE study achieving ACR20, -50 or -70 responses (A) over 6 months for the per protocol (PP) population (n = 693 in the s.c. abatacept-treated group, n = 678 in the i.v. abatacept-treated group; non-responder analysis) [11] and (B) over 32 months for patients who entered the LTE (all patients received s.c. abatacept + MTX, n = 1372; as-observed analysis) [26]. Fig. 1b Copyright © 2011 by the American College of Rheumatology. Fig. 1b Copyright © 2012 by the American College of Rheumatology.
The ACQUIRE study compared s.c. vs i.v. abatacept in MTX-IR patients. (A) The estimated difference between the s.c. and i.v. treatment groups for ACR20 at month 6 in the PP population (primary endpoint) was 0.3% (95% CI −4.2, 4.8). (B) Not all patients reached later time points at the time of data analysis. Data on eight patients were excluded from all efficacy analyses due to site non-compliance with study procedures. Error bars represent 95% CI.