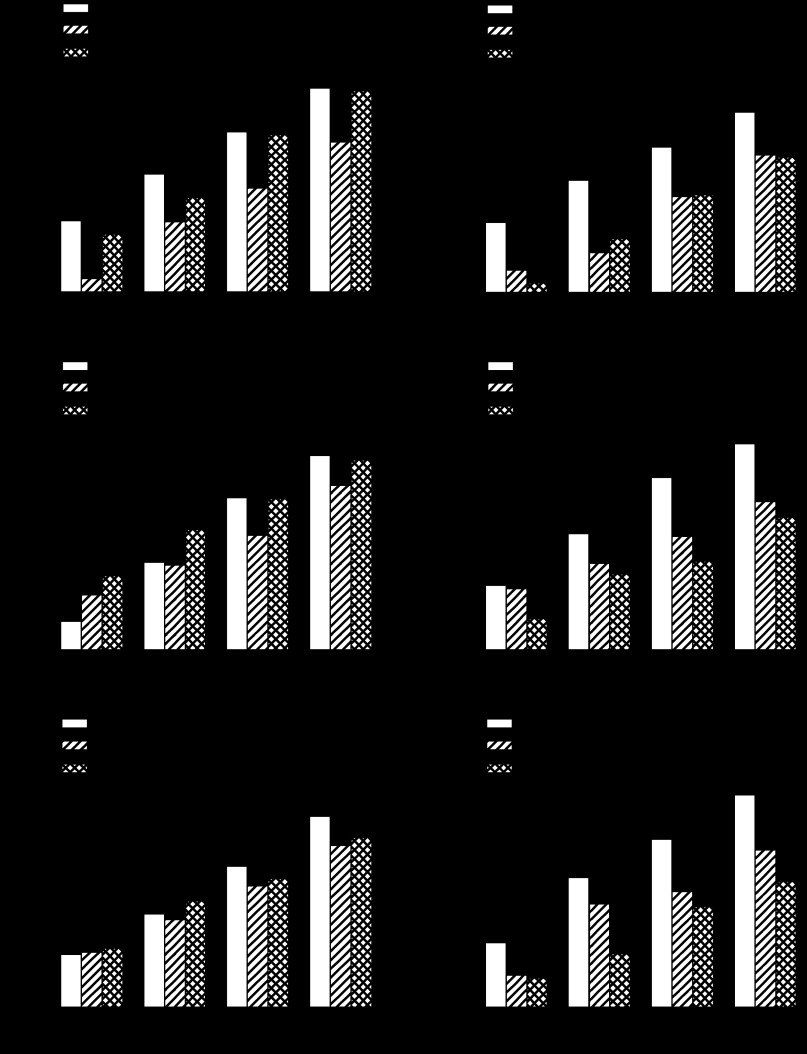Abstract
Peptide YY(3–36) [PYY(3–36)] is postulated to act as a hormonal signal from gut to brain to inhibit food intake. PYY(3–36) potently reduces food intake when administered systemically or into the brain. If action of endogenous PYY(3–36) is necessary for normal satiation to occur, then pharmacological blockade of its receptors should increase food intake. Here, we determined the effects of iv infusion of Y1, Y2, and Y5 receptor antagonists (BIBP 3226, BIIE 0246, CGP 71683) during the first 3 h of the dark period on food intake in non-food-deprived rats. Our results showed that 1) Y2 receptor blockade reversed the anorexic response to iv infusion of PYY(3–36) but did not increase food intake when administered alone; 2) Y1 and Y5 receptor antagonists neither attenuated PYY(3–36)-induced anorexia nor altered food intake when given alone; and 3) Y2 receptor blockade attenuated anorexic responses to gastric infusions of casein hydrolysate and long-chain triglycerides, but not maltodextrin. Previous work showed that Y2 antagonist BIIE 0246 does not penetrate the blood-brain barrier. Together, these results support the hypothesis that gut PYY(3–36) action at Y2 receptors peripheral to the blood brain barrier plays an essential role in mediating satiety responses to gastric delivery of protein and long-chain triglycerides, but not polysaccharide.
Keywords: PYY, Y receptor antagonists, BIIE 0246, protein, lipid, carbohydrate, food intake
peptide yy (pyy), neuropeptide y (NPY), and pancreatic polypeptide (PP) comprise a family of structurally-related brain-gut peptides with diverse actions mediated by five known receptors (Y1, Y2, Y4, Y5, Y6) (27). PYY, a 36-amino acid peptide, is synthesized in the gastrointestinal tract as well as in the central and peripheral nervous systems (12, 18, 19, 29). Endocrine cells of the ileum, colon, and rectum provide a major source of PYY (3). Food intake releases at least two major forms of PYY into the circulation: PYY(1–36) and PYY(3–36) (3, 22). PYY(1–36) binds to Y1, Y2, Y4, and Y5 receptors; PYY(3–36) is a high-affinity ligand for Y2 receptors with low affinity for Y1 and Y5 receptors (25). Systemic administration of PYY(3–36) potently inhibits food intake in humans (6), monkeys (30), and rodents (6, 15); PYY(1–36) is an order of magnitude less potent in humans (42) and rats (15). Pharmacological blockade of Y2 receptors reverses the anorexic response to systemic administration of PYY(3–36) (2, 38, 44). Thus, gut PYY(3–36) has been postulated to act at Y2 receptors to produce satiety (2).
If gut PYY(3–36) action at Y2 receptors is necessary for normal satiation to occur, then food intake should increase in response to knockout of either the PYY gene or Y2 receptor gene or to pharmacological blockade of Y2 receptors. Germline deletion of the PYY gene increased food intake, however, in just one of three studies (7, 9, 48). Deletion of the Y2 receptor gene also produced mixed results. One study reported an increase in food intake in both female and male mice (31), while another reported an increase in food intake in females and a transient decrease in males (37). Selective adult-onset deletion of Y2 receptors has been reported to increase food intake when deletion was confined to the hypothalamus (40) yet have no effect when localized to NPY neurons in the hypothalamus (40) or to tissues outside the brain (39). Potential explanations for these different outcomes include developmental compensation in knockout mice, different techniques employed to create knockouts, and strain differences.
Studies using pharmacological blockade of Y2 receptors have also produced mixed results. In rats, injection of Y2 receptor antagonist BIIE 0246 into the hypothalamic arcuate nucleus (ARC) blocked the anorexic response to intraperitoneal (ip) injection of PYY(3–36) and increased food intake in sated rats when administered alone during the light period, yet had no effect on feeding when administered at dark onset (2). Food intake also was not changed when either Y2 receptor antagonist BIIE 0246 or JNJ-31020028 was injected systemically at a dose that blocked PYY(3–36)-induced anorexia (38, 41, 44). It is not clear why these discrepancies occurred. If the Y2 receptor antagonist has a relatively short half-life, bolus dosing may not be sufficient to attenuate the satiety effects of a prolonged meal-induced secretion of PYY(3–36). Another possible reason is that Y2 receptor blockade is less able to increase food intake in previously food-deprived animals that already express a significant drive to eat. Furthermore, if PYY(3–36) is but one of several satiety signals produced by a specific meal, then Y2 receptor blockade alone may have little if any effect on ingestion of that meal.
Here, we systemically administered antagonists of PYY receptors to test the hypothesis that gut PYY(3–36) plays an essential role in mediating nutrient-induced satiety. Initial experiments determined the effects of short-term, intravenous (iv) infusions of Y1, Y2, and Y5 receptor antagonists (BIBP 3226, BIIE 0246, and CGP 71683, respectively) at dark onset on food intake in rats and on the anorexic response to iv infusion of PYY(3–36). Subsequent experiments determined the effects of iv infusion of Y2 receptor antagonist BIIE 0246 on anorexic responses to gastric infusions of casein hydrolysate, a mixture of long-chain triglycerides, and maltodextrin. We reasoned that gastric infusion of the macronutrients might reduce a “ceiling effect” that limits the expression of an orexigenic response to Y2 receptor blockade in hungry rats. Using this approach, we previously provided evidence that the gut peptide cholecystokinin and the pancreatic peptide amylin play essential roles in mediating nutrient-induced satiety (34, 35, 45–47).
METHODS
Subjects.
Male rats (Sasco Sprague Dawley, Charles River Laboratories, Kingston, NY; ∼350 g at start of study) were housed individually in hanging wire-mesh cages in a temperature-controlled room with a 12:12-h light-dark cycle (lights off at 1600). Animals were provided rat chow (Labdiet, 5001 Rodent diet, 3.3 kcal/g; PMI Nutrition International, Brentwood, MO) and water ad libitum. The Animal Studies Subcommittee of the Omaha Veterans Affairs Medical Center approved the experimental protocol.
Surgical procedures.
Procedures for implanting a gastric catheter for nutrient infusion and a jugular vein catheter for administration of PYY(3–36) and Y1, Y2, and Y5 receptor antagonists were described previously (34, 45). Gastric and jugular vein catheters were filled with water and heparinized saline (40 U/ml), respectively, plugged with stainless steel wire, and flushed every other day to maintain patency. Catheters were connected to 40-cm lengths of tubing passed through a protective spring coil, connected between a lightweight saddle worn by the rat and either a single- or double-channel infusion swivel (Instech Laboratories, Plymouth Meeting, PA). The double-channel swivel permitted simultaneous administration of Y2 antagonist intravenously and macronutrient intragastrically.
PYY(3–36) and Y1, Y2, and Y5 receptor antagonists.
Rat PYY(3–36) was synthesized by Fmoc solid-phase methodology (4) and purified by reverse-phase high-performance liquid chromatography. Proof of structure was provided by electrospray mass spectrometry. BIBP 3226 (Y1 antagonist), BIIE 0246 (Y2 antagonist), and CGP 71683 (Y5 antagonist) were purchased from Tocris Bioscience (R&D Systems, Minneapolis, MN).
Effects of Y1, Y2, and Y5 receptor antagonists on food intake and PYY(3–36)-induced anorexia.
Three experiments were performed. The first experiment determined the effects of iv infusion of Y1 antagonist BIBP 3226 (3,000 pmol · kg−1 · min−1) on feeding and on the anorexic response to iv infusion of PYY(3–36) (30 pmol · kg−1 · min−1). This PYY(3–36) dose is approximately twice the mean effective dose of PYY(3–36) for inhibiting food intake in rats under the same feeding conditions (15). This dose was chosen because it significantly reduced 3-h food intake by ∼40%. The second and third experiments of similar design determined the effects of iv infusions of Y2 antagonist (BIIE 0246) and Y5 antagonist (CGP 71683) on feeding and on the anorexic response to PYY(3–36) infusion. Two different sets of rats were used for these experiments. One set was used for the Y1 receptor antagonist; the other set was used for the Y2 and Y5 receptor antagonists.
Animals were permitted at least 1 wk to recover from surgery. They were then tethered to infusion swivels and adapted to experimental conditions for at least 1 wk before start of experiments. Excess amounts of fresh ground rat chow were provided each day at 1300. In the first experiment, non-food-deprived rats (n = 16) received a 3.75-h iv infusion of BIBP 3226 (3,000 pmol · kg−1 · min−1; 1.5 ml/h) or vehicle [2% dimethylsulfoxide (DMSO); 1% Tween 80, 97% 0.15 M NaCl, 0.1% bovine serum albumen (BSA)] beginning 30 min before receiving a 3-h iv infusion of PYY(3–36) (30 pmol · kg−1 · min−1; 1.5 ml/h) or vehicle (0.15 M NaCl, 0.1% BSA), which began 15 min before dark onset. BIBP 3226 infusion was begun 30 min before PYY(3–36) infusion to give BIBP 3226 a competitive advantage in binding to Y1 receptors. Cumulative hourly food intake during the first 4 h after dark onset was determined, as described previously, from continuous computer recordings of changes in food bowl weight (45). Infusions were administered using a syringe infusion pump (Harvard Apparatus, South Natick, MA); pumps were turned on and off by computer program. Each rat received each treatment in random order at intervals of at least 48 h. At the end of the experiment, data from a rat were excluded if its jugular vein catheter was not patent. A catheter was deemed patent if the rat lost consciousness within 10 s of a bolus injection of the short-acting anesthetic brevital into the catheter. In the second and third experiments of similar design, rats (n = 16) received iv infusions of either Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) and PYY(3–36) (30 pmol · kg−1 · min−1) or Y5 antagonist CGP 71683 (3,000 pmol · kg−1 · min−1) and PYY(3–36) (30 pmol · kg−1 · min−1).
Effects of Y2 receptor blockade on feeding responses to gastric infusions of casein hydrolysate, long-chain triglycerides, and maltodextrin.
Six experiments were performed. Two experiments determined the effects of iv infusion of Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) on feeding responses to gastric infusion of casein hydrolysate [2 and 3 kcal/h, 4 ml/h, Tryptone (trypsin digestion of casein); Fisher Scientific]. Treatments were administered to groups of 16 rats as above for the PYY(3–36) experiments, except that Tryptone was infused into the stomach for 2 h beginning 15 min before dark onset. Two experiments of identical design determined the effects of Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) on feeding responses to gastric infusion of a mixture of long-chain triglycerides (4 and 6 kcal/h, 2 and 3 ml/h, respectively, Liposyn II; Hospira, Lake Forest, IL). Liposyn II contains 10% safflower oil, 10% soybean oil, 1.2% egg phosphatides, and 2.5% glycerin in water (2 kcal/ml); major triglyceride fatty acids were 65.8% linoleic, 17.7% oleic, 8.8% palmitic, 3.4% stearic, and 4.2% linolenic acid. Two experiments of identical design determined the effects of Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) on feeding responses to 2-h gastric infusion of maltodextrin (2 and 4 kcal/h, 4 ml/h, Polycose; Abbott Nutrition, Columbus, OH). Water was used as vehicle and diluent for Tryptone and Polycose, while saline was used for Liposyn II. Two different sets of rats were used for these experiments. One set was used for the 2 kcal/h dose of Tryptone, 6 kcal/h dose of Liposyn II, and 4 kcal/h dose of Polycose. A second set was used for the 3 kcal/h dose of Tryptone, 4 kcal/h dose of Liposyn II, and 2 kcal/h dose of Polycose. Based on our similar studies using duodenal nutrient infusions (34, 35), these macronutrient doses were predicted to produce a 25–50% reduction in food intake during the first few hours of the dark period. Yiin et al. (49) have provided evidence that, in rats, similar gastric rates of infusion of Polycose, corn oil, and casein produce learned flavor preferences rather than aversions, suggesting that they do not reduce food intake by producing malaise.
Statistical analyses.
Values are presented as group means ± SE. Effects of Y receptor antagonists on feeding, PYY(3–36)-induced inhibition of feeding, and feeding responses to gastric nutrient infusions were evaluated by repeated-measures ANOVA. Feeding data included cumulative hourly food intake during the 4-h test period. Planned comparisons of treatment means were evaluated by paired t-tests. Differences were considered significant if P < 0.05.
RESULTS
Effects of Y1, Y2, and Y5 receptor antagonists on food intake and PYY(3–36)-induced anorexia.
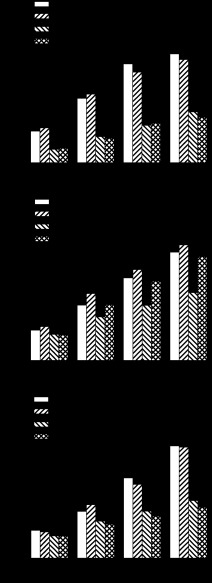
Figure 1A shows the individual and combined effects of iv infusions of Y1 antagonist BIBP 3226 (3,000 pmol · kg−1 · min−1) and PYY(3–36) (30 pmol · kg−1 · min−1) on food intake during the early dark period. ANOVA showed a significant main effect of the 3-h infusion of PYY(3–36) on cumulative food intake at 1, 2, 3, and 4 h after infusion onset and no significant main effect of Y1 antagonist or interaction of Y1 antagonist and PYY(3–36) on cumulative intake at any time point. Comparisons of individual treatment means showed that PYY(3–36) significantly reduced cumulative intake at 1, 2, 3, and 4 h after infusion onset by 58, 59, 62, and 53% compared with the response to vehicle infusion. Y1 antagonist neither attenuated PYY(3–36)-induced anorexia nor altered food intake when given alone.
Fig. 1.
Effects of Y1 receptor antagonist (BIBP 3226; A), Y2 receptor antagonist (BIIE 0246; B), and Y5 receptor antagonist (CGP 71683; C) on food intake and PYY(3–36)-induced anorexia. Non-food-deprived rats (n = 16) received a 3.75-h iv infusion of receptor antagonist (3,000 pmol · kg−1 · min−1) or vehicle beginning 30 min before receiving a 3-h iv infusion of PYY(3–36) (30 pmol · kg−1 · min−1) or vehicle, which began 15 min before dark onset. Food intake was measured during the first 4 h after dark onset. Values are means ± SE. Treatment means labeled with the same letter are not statistically different (P < 0.05).
Figure 1B shows the individual and combined effects of iv infusions of Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) and PYY(3–36) (30 pmol · kg−1 · min−1) on food intake. ANOVA showed significant main effects of Y2 antagonist and PYY(3–36) on cumulative food intake at 2, 3, and 4 h and a significant interaction of Y2 antagonist and PYY(3–36) on cumulative intake at 4 h. Comparisons of individual treatment means showed that PYY(3–36) significantly reduced cumulative intake at 2, 3, and 4 h by 21, 33, and 37%, respectively. Y2 antagonist significantly reversed PYY(3–36)-induced anorexia at 2, 3, and 4 h by 53, 68, and 75%, respectively, yet had no significant effect on food intake when given alone.
Figure 1C shows the individual and combined effects of iv infusions of Y5 antagonist CGP 71683 (3,000 pmol · kg−1 · min−1) and PYY(3–36) (30 pmol · kg−1 · min−1) on food intake during the early dark period. ANOVA showed a significant main effect of PYY(3–36) on cumulative food intake at 2, 3, and 4 h and no significant main effect of Y5 antagonist or interaction of Y5 antagonist and PYY(3–36) on cumulative intake at any time point. Comparisons of individual treatment means showed that PYY(3–36) significantly reduced cumulative intake at 1, 2, 3, and 4 h after infusion onset by 20, 21, 41, and 49%, respectively. Y5 antagonist neither attenuated PYY(3–36)-induced anorexia nor altered food intake when given alone.
Effects of Y2 receptor blockade on feeding responses to gastric infusions of casein hydrolysate, long-chain triglycerides, and maltodextrin.
Figure 2, A and B, shows the effects of iv infusion of Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) on anorexic responses to 2-h gastric infusions of casein hydrolysate (4 and 6 kcal of Tryptone) during the early dark period. The 4-kcal dose of Tryptone significantly reduced cumulative intake at 1, 2, 3, and 4 h by 81, 41, 35, and 26%, respectively, compared with the response to vehicle infusion. Y2 antagonist significantly reversed Tryptone-induced anorexia at 1, 3, and 4 h by 77, 95, and 95%, respectively (Fig. 2A). The 6-kcal dose of Tryptone significantly reduced cumulative intake at 1, 2, 3, and 4 h by 68, 64, 34, and 24%, respectively; Y2 antagonist did not attenuate Tryptone-induced anorexia at any time point (Fig. 2B).
Fig. 2.
Effects of Y2 receptor antagonist (BIIE 0246) on anorexic responses to gastric infusions of different doses of casein hydrolysate (Tryptone; A and B), a mixture of long-chain triglycerides (Liposyn II; C and D), and maltodextrin (Polycose; E and F). Non-food-deprived rats (n = 12–16) received a 3.75-h iv infusion of Y2 antagonist (3,000 pmol · kg−1 · min−1) or vehicle beginning 30 min before receiving a 2-h gastric infusion of macronutrient, which began 15 min before dark onset. Food intake was measured during the first 4 h after dark onset. Values are means ± SE. Treatment means labeled with the same letter are not statistically different (P < 0.05).
Figure 2, C and D, shows the effects of iv infusion of Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) on anorexic responses to 2-h gastric infusions of a mixture of long-chain triglycerides (8 and 12 kcal of Liposyn II). The 8-kcal dose of Liposyn significantly reduced cumulative intake at 3 and 4 h by 25 and 15%, respectively; Y2 antagonist significantly reversed Liposyn-induced anorexia at 3 h by 97% (Fig. 2C). The 12-kcal dose of Liposyn significantly reduced cumulative intake at 2, 3, and 4 h by 26, 34, and 28%, respectively; Y2 antagonist did not attenuate Liposyn-induced anorexia at any time point (Fig. 2D).
Figure 2, E and F, shows the effects of iv infusion of Y2 antagonist BIIE 0246 (3,000 pmol · kg−1 · min−1) on anorexic responses to 2-h gastric infusions of maltodextrin (4 and 8 kcal of Polycose). The 4-kcal dose of Polycose did not significantly reduce cumulative intake at any time (Fig. 2E). The 8-kcal dose of Polycose significantly reduced cumulative intake at 1, 3, and 4 h by 50, 31, and 26%, respectively; Y2 antagonist did not attenuate Polycose-induced anorexia at any time point (Fig. 2F).
DISCUSSION
Here, we systemically administered antagonists of Y receptors to test the hypothesis that gut PYY(3–36) plays an essential role in mediating nutrient-induced satiety. We determined the effects of iv infusion of Y1, Y2, and Y5 receptor antagonists (BIBP 3226, BIIE 0246, and CGP 71683, respectively) during the first 3 h of the dark period on food intake in non-food-deprived rats. Our results showed that 1) Y2 receptor blockade reversed the anorexic response to iv infusion of PYY(3–36) but did not increase food intake when administered alone; 2) Y1 and Y5 receptor antagonists neither attenuated PYY(3–36)-induced anorexia nor altered food intake when given alone; and 3) Y2 receptor blockade attenuated anorexic responses to gastric infusions of lower doses of casein hydrolysate and long-chain triglycerides but not to maltodextrin infusions. Previous work showed that Y2 antagonist BIIE 0246 does not penetrate the blood-brain barrier (13). Together, these results support the hypothesis that gut PYY(3–36) action at Y2 receptors peripheral to the blood-brain barrier plays an essential role in mediating satiety responses to gastric delivery of protein and long-chain triglycerides but not polysaccharide.
Previous studies reported no change in food intake in rodents when either Y2 receptor antagonist BIIE 0246 or JNJ-31020028 was injected systemically at a dose that blocked PYY(3–36)-induced anorexia (38, 41, 44). However, if the antagonists have a relatively short half-life, their bolus dosing may not have been sufficient to attenuate the satiety effects of a prolonged meal-induced secretion of PYY(3–36). Y2 receptor blockade also may not have been able to significantly increase food intake if antagonists were administered to animals that already expressed a significant drive to eat. Indeed, in the present study, a 3-h iv infusion of Y2 receptor antagonist that reversed PYY(3–36)-induced anorexia did not increase food intake when administered alone to rats during the early dark period of active food intake. In an attempt to obviate these possible problems in the present study, we examined the effects of prolonged iv infusion of the Y2 receptor antagonist BIIE 0246 on anorexic responses to gastric nutrient infusions. Our results suggest that the nutrient infusions may have reduced a “ceiling effect” that limited the expression of an orexigenic response to Y2 receptor blockade in the hungry rats. We previously provided evidence using this approach that the gut peptide cholecystokinin and the pancreatic peptide amylin play essential roles in mediating nutrient-induced satiety (34, 35, 45–47).
In the present study Y2 receptor blockade attenuated anorexic responses to gastric infusions of lower but not higher doses of casein hydrolysate and long-chain triglycerides. We observed a similar inverse relationship between orexigenic responses to CCK receptor blockade and rate of macronutrient delivery to the gastrointestinal tract (35, 45–47). Together, these results suggest that gut peptides PYY(3–36) and CCK each play an essential role in mediating the satiety response to lower rates of nutrient delivery to the gastrointestinal tract and that higher delivery rates stimulate redundant satiety mechanisms. For example, in the present study, higher rates of nutrient delivery to the stomach may have produced higher rates of secretion of gastrointestinal peptides CCK, amylin, and glucagon-like peptide-1, which further inhibited gastric emptying to produce a greater gastric distention-induced satiation that was resistant to Y2 receptor blockade (14, 33, 36). Thus, discrepancies among studies examining the effects of Y2 receptor blockade on food intake may have been due in part to differences in redundancy of satiety signaling produced by the different experimental meals employed.
Here, we show that peripheral administration of a Y2 receptor antagonist attenuated anorexic responses to gastric infusions of casein hydrolysate and long-chain triglycerides, but not maltodextrin. A possible explanation for this is that intragastric infusions of maltodextrin stimulated redundant satiety signaling to a greater degree than the lower doses of casein hydrolysate and lipid, such that Y2 receptor blockade had no effect on maltodextrin-induced anorexia. Other studies suggest that gastrointestinal delivery of protein and lipid increase plasma PYY levels to a greater degree than carbohydrate, although results varied considerably across species, treatment conditions, and sites of nutrient delivery [(7, 16, 17, 20, 21, 24, 28); see also Refs. 10 and 43]. A specific role for PYY in mediating the satiating effects of high-protein diets is supported by evidence showing that high-protein diet-induced anorexia is attenuated in PYY-null mice (7). Our work here extends these findings to suggest that gut PYY(3–36) plays an essential role in mediating satiety responses to gastrointestinal delivery of protein and long-chain triglycerides but not polysaccharide.
The mechanism through which PYY(3–36) reduces food intake remains to be determined. A direct action of circulating PYY(3–36) at Y2 receptors in the ARC has been proposed (2, 6). Supporting evidence includes that 1) iv injected PYY(3–36) penetrates the blood-brain barrier in mice (32); 2) ARC neurons possess Y2 receptors in rodents (11); 3) injection of PYY(3–36) into the ARC reduces food intake in rats (6); 4) injection of Y2 receptor antagonist BIIE 0246 into the ARC blocks the anorexic response to ip injection of PYY(3–36) and increases food intake in sated rats (2); and 5) we previously showed that iv infusion of PYY(3–36) at the dose used in the present study increases Fos, an indicator of neural activation, in the ARC of rats (8).
Other studies support the hypothesis that gut PYY(3–36) acts at Y2 receptors peripheral to the blood-brain barrier to reduce food intake. Supporting evidence includes the following: 1) subcutaneous injection of PYY(3–36) conjugated to albumen reduces food intake in rats despite its inability to penetrate the blood-brain barrier or to increase Fos expression in the ARC (5); and 2) vagal afferent neurons possess Y2 receptors (26), and subdiaphragmatic vagotomy attenuates PYY(3–36)-induced anorexia in rats (1, 26), although not in mice (23). The present study further supports this hypothesis. Intravenous infusion of Y2 receptor antagonist BIIE 0246, which does not readily penetrate the blood-brain barrier (13), attenuated anorexic responses to iv infusion of PYY(3–36) and to gastric infusions of casein hydrolysate and long-chain triglycerides but not maltodextrin. Together, these results suggest that gut PYY(3–36) action at Y2 receptors peripheral to the blood-brain barrier plays an essential role in mediating satiety responses to gastric delivery of protein and long-chain triglycerides but not polysaccharide. The relative importance of peripheral and central Y2 receptors in mediating nutrient-induced satiety remains to be determined.
GRANTS
This work was performed at the VA Nebraska-Western Iowa Health Care System, and was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service, and Biomedical Laboratory Research and Development. This research was also supported in part by the National Institutes of Health (R01 DK-73152 and P20 RR-16469). Contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.D.R., A.C.H., and P.K.C. conception and design of research; R.D.R., A.C.H., and P.K.C. performed experiments; R.D.R., A.C.H., and P.K.C. analyzed data; R.D.R., A.C.H., and P.K.C. interpreted results of experiments; R.D.R. prepared figures; R.D.R. drafted manuscript; R.D.R., A.C.H., and P.K.C. edited and revised manuscript; R.D.R., A.C.H., and P.K.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Krista Anders, Bettye Apenteng, Sharalyn Steenson, James Buescher, Linda Kelsey, and Dean Heiman for excellent technical assistance.
REFERENCES
- 1. Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res 1043: 139–144, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077, 1985 [DOI] [PubMed] [Google Scholar]
- 4. Amblard M, Fehrentz JA, Martinez J, Subra G. Methods and protocols of modern solid phase peptide synthesis. Mol Biotechnol 33: 239–254, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Baraboi ED, Michel C, Smith P, Thibaudeau K, Ferguson AV, Richard D. Effects of albumin-conjugated PYY on food intake: the respective roles of the circumventricular organs and vagus nerve. Eur J Neurosci 32: 826–839, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4: 223–233, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Blevins JE, Chelikani PK, Haver AC, Reidelberger RD. PYY(3–36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides 29: 112–119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia 49: 1360–1370, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol 303: G129–G140, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Broberger C, Landry M, Wong H, Walsh JN, Hökfelt T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology 66: 393–408, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Broome M, Hokfelt T, Terenius L. Peptide YY (PYY)-immunoreactive neurons in the lower brain stem and spinal cord of rat. Acta Physiol Scand 125: 349–352, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Brothers SP, Saldanha SA, Spicer TP, Cameron M, Mercer BA, Chase P, McDonald P, Wahlestedt C, Hodder PS. Selective and brain penetrant neuropeptide Y Y2 receptor antagonists discovered by whole-cell high-throughput screening. Mol Pharmacol 77: 46–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288: R1695–R1706, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology 146: 879–888, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Dailey MJ, Tamashiro KL, Terrillion CE, Moran TH. Nutrient specific feeding and endocrine effects of jejunal infusions. Obesity (Silver Spring) 18: 904–910, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Degen L, Drewe J, Piccoli F, Gräni K, Oesch S, Bunea R, D'Amato M, Beglinger C. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. Am J Physiol Regul Integr Comp Physiol 292: R1391–R1399, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides 23: 251–261, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Ekman R, Wahlestedt C, Bottcher G, Sundler F, Hakanson R, Panula P. Peptide YY-like immunoreactivity in the central nervous system of the rat. Regul Pept 16: 157–168, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab 92: 4052–4055, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Feinle-Bisset C, Patterson M, Ghatei MA, Bloom SR, Horowitz M. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab 289: E948–E953, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1–36 and PYY 3–36. Regul Pept 51: 151–159, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Helou N, Obeid O, Azar ST, Hwalla N. Variation of postprandial PYY 3–36 response following ingestion of differing macronutrient meals in obese females. Ann Nutr Metab 52: 188–195, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Keire DA, Bowers CW, Solomon TE, Reeve JR. Structure and receptor binding of PYY analogs. Peptides 23: 305–321, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, Nakazato M. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology 146: 2369–2375, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 50: 143–150, 1998 [PubMed] [Google Scholar]
- 28. Min DK, Tuor UI, Koopmans HS, Chelikani PK. Changes in differential functional magnetic resonance signals in the rodent brain elicited by mixed-nutrient or protein-enriched meals. Gastroenterology 141: 1832–1841, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Miyachi Y, Jitsuishi W, Miyoshi A, Fujita S, Mizuchi A, Tatemoto K. The distribution of polypeptide YY-like immunoreactivity in rat tissues. Endocrinology 118: 2163–2167, 1986 [DOI] [PubMed] [Google Scholar]
- 30. Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE. Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 288: R384–R388, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, Ernfors P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med 5: 1188–1193, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Nonaka N, Shioda S, Niehoff ML, Banks WA. Characterization of blood-brain barrier permeability to PYY3–36 in the mouse. J Pharmacol Exp Ther 306: 948–953, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Reidelberger RD, Arnelo U, Granqvist L, Permert J. Comparative effects of amylin and cholecystokinin on food intake and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 280: R605–R611, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J. Amylin receptor blockade stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol 287: R568–R574, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Reidelberger RD, Heimann D, Kelsey L, Hulce M. Effects of peripheral CCK receptor blockade on feeding responses to duodenal nutrient infusions in rats. Am J Physiol Regul Integr Comp Physiol 284: R389–R398, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Reidelberger RD, Kelsey L, Heimann D. Effects of amylin-related peptides on food intake, meal patterns, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 282: R1395–R1404, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, Cox HM, Sperk G, Hökfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA 99: 8938–8943, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3–36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol 17: 452–457, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Shi YC, Lin S, Castillo L, Aljanova A, Enriquez RF, Nguyen AD, Baldock PA, Zhang L, Bijker MS, Macia L, Yulyaningsih E, Zhang H, Lau J, Sainsbury A, Herzog H. Peripheral-specific y2 receptor knockdown protects mice from high-fat diet-induced obesity. Obesity (Silver Spring) 19: 2137–2148, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Shi YC, Lin S, Wong IP, Baldock PA, Aljanova A, Enriquez RF, Castillo L, Mitchell NF, Ye JM, Zhang L, Macia L, Yulyaningsih E, Nguyen AD, Riepler SJ, Herzog H, Sainsbury A. NPY neuron-specific Y2 receptors regulate adipose tissue and trabecular bone but not cortical bone homeostasis in mice. PLoS One 5: e11361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shoblock JR, Welty N, Nepomuceno D, Lord B, Aluisio L, Fraser I, Motley ST, Sutton SW, Morton K, Galici R, Atack JR, Dvorak L, Swanson DM, Carruthers NI, Dvorak C, Lovenberg TW, Bonaventure P. In vitro and in vivo characterization of JNJ-31020028 [N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2-pyridin-3-ylbenzamide], a selective brain penetrant small molecule antagonist of the neuropeptide Y Y(2) receptor. Psychopharmacology (Berl) 208: 265–277, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol 292: E1062–E1068, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Steinert RE, Meyer-Gerspach AC, Beglinger C. The role of the stomach in the control of appetite and the secretion of satiation peptides. Am J Physiol Endocrinol Metab 302: E666–E673, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Woltman T, Castellanos D, Reidelberger R. Role of cholecystokinin in the anorexia produced by duodenal delivery of oleic acid in rats. Am J Physiol Regul Integr Comp Physiol 269: R1420–R1433, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Woltman T, Reidelberger R. Role of cholecystokinin in the anorexia produced by duodenal delivery of glucose in rats. Am J Physiol Regul Integr Comp Physiol 271: R1521–R1528, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Woltman T, Reidelberger R. Role of cholecystokinin in the anorexia produced by duodenal delivery of peptone in rats. Am J Physiol Regul Integr Comp Physiol 276: R1701–R1709, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Wortley KE, Garcia K, Okamoto H, Thabet K, Anderson KD, Shen V, Herman JP, Valenzuela D, Yancopoulos GD, Tschöp MH, Murphy A, Sleeman MW. Peptide YY regulates bone turnover in rodents. Gastroenterology 133: 1534–1543, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Yiin YM, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and free-feeding rats. Physiol Behav 84: 217–231, 2005 [DOI] [PubMed] [Google Scholar]