Abstract
Purpose
Numerous studies have investigated causes of warfarin dose variability in adults, whereas studies in children are limited both in numbers and size. Mechanism-based population modelling provides an opportunity to condense and propagate prior knowledge from one population to another. The main objectives with this study were to evaluate the predictive performance of a theoretically bridged adult warfarin model in children, and to compare accuracy in dose prediction relative to published warfarin algorithms for children.
Method
An adult population pharmacokinetic/pharmacodynamic (PK/PD) model for warfarin, with CYP2C9 and VKORC1 genotype, age and target international normalized ratio (INR) as dose predictors, was bridged to children using allometric scaling methods. Its predictive properties were evaluated in an external data set of children 0–18 years old, including comparison of dose prediction accuracy with three pharmacogenetics-based algorithms for children.
Results
Overall, the bridged model predicted INR response well in 64 warfarin-treated Swedish children (median age 4.3 years), but with a tendency to overpredict INR in children ≤2 years old. The bridged model predicted 20 of 49 children (41 %) within ± 20 % of actual maintenance dose (median age 7.2 years). In comparison, the published dosing algorithms predicted 33–41 % of the children within ±20 % of actual dose. Dose optimization with the bridged model based on up to three individual INR observations increased the proportion within ±20 % of actual dose to 70 %.
Conclusion
A mechanism-based population model developed on adult data provides a promising first step towards more individualized warfarin therapy in children.
Electronic supplementary material
The online version of this article (doi:10.1007/s00228-012-1466-4) contains supplementary material, which is available to authorized users.
Keywords: PK/PD model, Population analysis, Warfarin, Dosing, Children
Introduction
Although anticoagulant therapy is not common in children, it is crucial for specific patient groups, for example children with congenital or acquired heart diseases [1]. The most commonly prescribed anticoagulant in both adults and children is warfarin [2]. Warfarin therapy is challenging in general, but even more so in children, partly due to the lack of age-appropriate formulations for accurate and reproducible dosing. Manipulation of warfarin tablets before administration is common practice and may lead to inconsistent dosing [3]. In addition, warfarin has a narrow therapeutic range and a considerable variability in individual dose requirements. In adult Caucasian patients, over 40 % of the inter-individual variability in dose is explained by polymorphisms in two genes, VKORC1 and CYP2C9 [4]. Several pharmacogenetics-guided dosing algorithms have been developed for adults [4–8], and some are currently being tested in clinical trials [9–11]. Only a few small studies have investigated the influence of clinical and genetic factors on warfarin dose in children [12–14]. In the studies by Moreau et al. (n = 84) [13] and Biss et al. (n = 120) [14], clinical and genetic factors explained 70 % of the dose variability, but less than 30 % in the study by Nowak-Göttl et al. (n = 34) [12].
For many medicinal products, dosing recommendations for children are based on extrapolation of results from adults. When scaling adult doses to children, linear predictions of dose based on body weight are commonly used, so also for warfarin [1, 12]. However, it is becoming more widely recognized that the relationship between adult and paediatric doses is often non-linear. Due to a larger liver and/or kidneys in relation to body size, small children often require higher doses per kilo bodyweight than older children and adults to achieve the same target concentration [15]. This has also been observed with warfarin, where a higher weight-normalized dose was required in the youngest age-groups to maintain the same target international normalized ratio (INR) [14, 16, 17].
A growing amount of data supports that the pharmacokinetics of drugs in children ≥2 years can be reasonably well extrapolated from adults by using an allometric adjustment for body size [15, 18]. In the youngest age group (< 2 years), it is also necessary to take into account the impact of maturation of the organs and/or enzymes involved in elimination of the drug under study. Hence, when predicting the pharmacokinetics of drugs in children 0–18 years old, it is important to acknowledge the influence of both body size and age. Whether this will be sufficient for accurate dose predictions will also depend on if the pharmacodynamics in children can be assumed to be the same as in adults.
Pharmacometric models developed on adult data are increasingly being used to predict dosing in children [19, 20]. We have developed a pharmacokinetic/pharmacodynamic (PK/PD)-based population model for warfarin that describes the relationship between dose and INR response in adults [21, 22]. This model takes into account variation in pharmacokinetics due to age and CYP2C9 genotype and variation in pharmacodynamics due to VKORC1 genotype. However, the existing model does not include a size measure such as weight, which is necessary when considering allometric scaling of an adult model for children. Modest revisions of the existing model were therefore necessary to enable bridging to children.
The main objectives of the present study were (1) to reformulate an existing adult model for warfarin to enable bridging to children, (2) to evaluate how well a theoretically scaled adult PK/PD-based warfarin model predicts the dose-INR relationship in a cohort of warfarin-treated children, and (3) to compare the accuracy of maintenance dose prediction between this new warfarin model and three published pharmacogenetics-based dosing algorithms for children [12–14].
Methods
Pharmacometric bridging of adult model to children
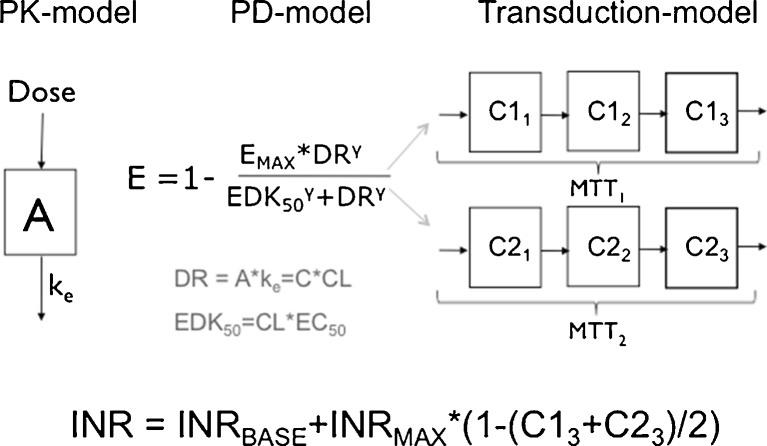
This work is built on a mechanism-based adult population model for warfarin that is founded on pharmacokinetic (PK) and pharmacodynamic (PD) principles. Figure 1 provides a schematic picture of the model, and for a more detailed description the reader is referred to Online Resource 1, or to the original publication [22]. On a population level, the model is a PK/PD-model, but when applied on an individual level, the lack of PK observations changes the scope of the model to a more empiric kinetic-pharmacodynamic (KPD) model. A detailed description of the model revisions that were undertaken to allow pharmacometric bridging to children is provided in Online Resource 1. In brief, the model revisions included re-estimation of model parameters after addition of allometric weight scaling on the PK parameters clearance (CL) and volume of distribution (V), and addition of a maturation function for the ontogeny of metabolising enzymes in the youngest age group (Eq. 1–3).
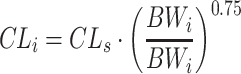 |
1 |
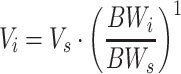 |
2 |
Fig. 1.
Schematic picture of the PK/PD-based population model for warfarin
CLi and Vi represent the typical parameter values in an individual with the body weight BWi, and CLs and Vs represent the typical parameter values in an individual with the body weight BWs.
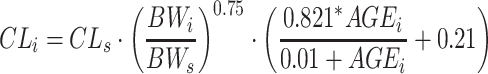 |
3 |
AGEi is the postnatal age expressed in years of an individual with the body weight BWi. The maturation function gives the fraction of the adult CL for a given age, where 100 % of the adult value is typically reached at 6 months.
The revised model is from now on referred to as the bridged KPD model.
Paediatric validation data set
Study subjects
Children were recruited from four tertiary care centres in Sweden: The Queen Silvia Children’s Hospital, Sahlgrenska University Hospital in Gothenburg; Children's Heart Center, Skåne University Hospital in Lund; Uppsala University Children’s Hospital in Uppsala and Astrid Lindgren Children’s Hospital in Stockholm. The study was approved by the Regional Ethical Review Board, Uppsala University, Uppsala, Sweden. Patients who were currently receiving or previously had received warfarin while below the age of 18 years were eligible. Written informed consent was obtained from each patient aged 18 years or over and from parents/guardians of children < 18 years in accordance with the Declaration of Helsinki. All children aged 7 years or older also provided written or verbal assent.
Collected treatment data
Information on warfarin doses and INRs were collected retrospectively from hospital records. Details on type of warfarin formulation (marketed Waran® 2.5 mg tablets from Nycomed or extemporaneously prepared 0.3 mg or 0.5 mg capsules) and INR method (Owren-based if monitored at hospitals and Quick-based if using a self-monitoring device) were recorded. Treatment histories were also collected prospectively until end of therapy or study closure (December 31, 2011). Treatment indication, target INR, date of birth, height and weight were extracted from patient charts. Both height and weight were treated as continuous variables, and this was achieved by linear interpolation between observations. Information on ethnicity (Caucasian, Asian, African or other/mix as alternatives), diet (formula-fed vs. non-formula-fed) and mode of warfarin administration [formulation administered intact (whole or divided) or after manipulation e.g. crushed and/or dissolved in food or liquids] was self-reported by parents/patients in a questionnaire, together with information on the approximate time of dosing and INR sampling.
Genotyping
All children were genotyped for single nucleotide polymorphisms (SNPs) in genes shown to be associated with warfarin dose in adults: I) CYP2C9—the gene coding for the enzyme involved in the metabolism of S-warfarin, and II) VKORC1—the gene coding for the target enzyme of warfarin. Details about the genotyping are provided in Online Resource 2.
Association of genetic variables with warfarin dose
The quantitative effect of CYP2C9 and VKORC1 genotype on warfarin maintenance dose in children was assumed to be the same as previously estimated for adults [22]. In short, this means that polymorphisms in CYP2C9 and VKORC1 each explain up to a 4.2-fold (CYP2C9 *3/*3 vs. *1/*1) and 2.1-fold (VKORC1 A/A vs. G/G) difference in warfarin maintenance dose.
Evaluation of model performance
Visual predictive checks (VPCs) were used to evaluate the predictive performance of the bridged KPD model on both the adult data set and the paediatric data set. The principle of a VPC is to graphically assess whether simulations from a model are able to reproduce both the central trend (the median curve) and the variability (outer percentiles) in the observed data when plotted against an independent variable, in this case, time. A prediction corrected VPC (pc-VPC) is a modification of the standard VPC that is suitable for evaluation of data collected in studies with an adaptive design [23], as is the case with warfarin. The pc-VPCs were constructed based on 500 simulated replicates of the original data set design. Results were presented as nonparametric 95 % confidence intervals for the median and outer percentiles. 2.5th and 97.5th percentiles were used for the adult data set, whereas 5th and 95th percentiles were used for the sparser paediatric data set. A model is considered to have good predictive properties if the lines representing the trend in the observed data (median and lower and upper percentiles) mainly fall inside the corresponding model based confidence intervals.
Comparison of dose prediction accuracy
The paediatric validation data set was also used to compare the accuracy in maintenance dose prediction between four different pharmacogenetic algorithms for warfarin dosing in children. Algorithms included were the bridged KPD model and three pharmacogenetics-based dose algorithms derived from regression analysis of paediatric data [12–14]. An empiric 0.2 mg/kg/day dose was also included as a non-pharmacogenetic reference regimen, although this represents a recommended starting dose rather than a maintenance dose [1].
The three published dose algorithms can all be used for a priori dose predictions, i.e. to predict the maintenance dose based on baseline values of the covariates. The bridged KPD model can provide such predictions as well. However, an advantage of the KPD model is that it can also be used for a posteriori predictions, i.e. individual dose revisions based on previous INR observations.
Children included in the comparison needed to fulfil the following criteria: 1) have complete genetic and clinical data for all dose algorithms, and 2) have a period of stable warfarin therapy according to the following criteria: (a) at least three consecutive INR measurements within target range over a minimum period of four weeks, and b) no change in dose during this time period.
Each model was used for a priori prediction of the daily maintenance dose. Table 1 provides an overview of individual predictors and the equations for published dose algorithms. All dose predictions with these algorithms were performed in Microsoft Excel, Version 12.3.2. Dose predictions with the bridged KPD model were performed in NONMEM, Version 7.2.0 [24], using the covariates specified in Table 1 and adopting the approach described by Jönsson and Karlsson [25]. This includes (i) defining a target steady-state response (in our case set to the midpoint of the target range, e.g. for a target range of 2.5-3.5, the target INR was set to 3.0); and (ii) predicting the dose required to achieve the target response, conditioned on the population (mean) parameter estimates.
Table 1.
Individual predictors included in the different pharmacogenetics-based warfarin dose algorithms for children
| Individual Predictors | A priori dose algorithms | A posteriori dose algorithm | |||
|---|---|---|---|---|---|
| Nowak-Göttl et al.a,d | Biss et al.b,e | Moreau et al.c,f | Bridged KPD model | Bridged KPD model | |
| Genetic factors | |||||
| CYP2C9 genotype | X | X | X | X | X |
| VKORC1 genotype | X | X | X | X | X |
| Demographics | |||||
| Age (years) | X | X | X | ||
| Weight (kg) | X | X | X | ||
| Height (cm) | X | X | |||
| Treatment related | |||||
| Baseline INR | X | X | |||
| Target INR | X | X | X | ||
| Indication | X | ||||
| Treatment history | |||||
| Previous Doses | Xg | ||||
| INR observations | Xg | ||||
a √Dose (mg/kg) = 0.49–0.013*Age(years) −0.08 (if VKORC1 A/A) + 0.01 (if VKORC1 A/G) −0.02 (if not CYP2C9 wild-type)
b √Dose (mg) = −0.009 + 0.0011*Height(cm) + 0.357*VKORC1 (0 for A/A, 1 for A/G, 2 for G/G) -0.478*M (M = number of CYP2C9*3 alleles) – 0.277*N (N = number of CYP2C9*2 alleles) + 0.186*Indication (0 for Fontan procedure, 1 for all other indications)
c Dose (mg/week) = −10.77 + 0.28*Height(cm) – 5.44*VKORC1 (0 for G/G, 1 for A/G, 2 for A/A) + 7.83 (if target INR 2.5) + 11.52 (if target INR 3.3) – 3.29*N (N = number of CYP2C9 *2 or *3 variant alleles)
d Algorithm derived on data from children with a target INR range of 2.0−3.0
e Algorithm derived on data from children with a target INR range of 2.0–3.0 or 2.5–3.5
f Algorithm derived on data from children with a target INR of 2.2 (1.5–3.3), 2.5 (1.8–3.2) or 3.3 (2.5–4.0)
g Previous doses and 1–3 INR observations from the period prior to the stable treatment period were used for the a posteriori prediction of maintenance dose.
For a posteriori dose predictions, the procedure was done in two steps. In the first step, each child’s treatment history, including up to three INR-observations from the period just prior to the stable treatment period, was used to derive individual parameter estimates. In the second step, the maintenance dose for each child was estimated as described under (i) and (ii) above, but now conditioned on the individual parameter estimates.
The output from each algorithm was converted to a predicted daily dose in mg, and compared with the actual average daily maintenance dose. Accuracy was assessed by calculating the difference between predicted and actual dose, with results expressed as the percentage prediction error [(predicted dose-observed dose)/observed dose*100]. Bias (mean prediction error) and imprecision (root mean square error) was calculated for each method. Clinical accuracy was assessed by calculating the proportion of patients in which the predicted dose was 1) within 20 % of the actual dose (ideal dose prediction); 2) at least 20 % below the actual dose (underprediction); or 3) at least 20 % above the actual dose (overprediction) [6].
Results
Revision of the adult KPD model
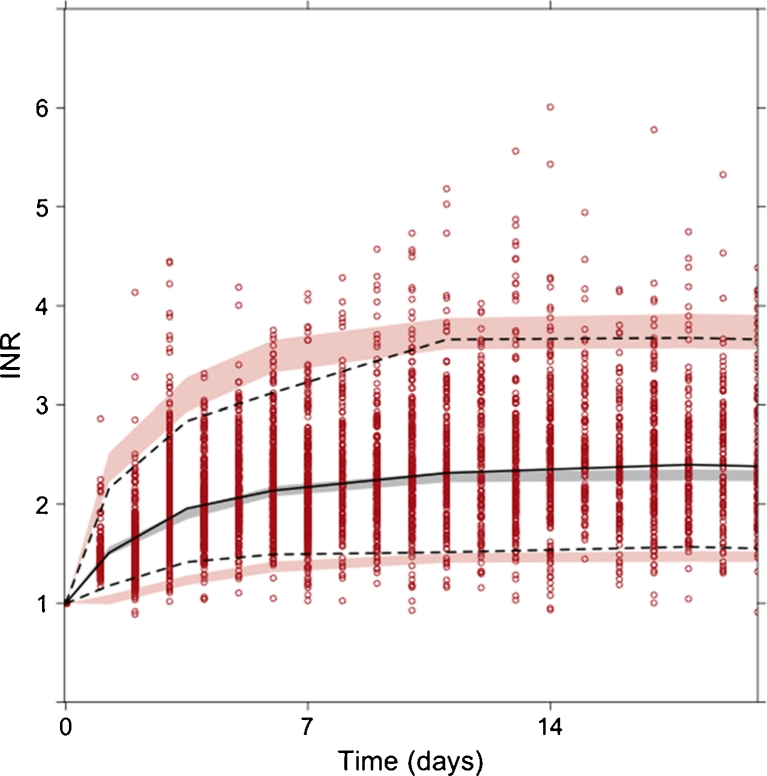
The revised adult KPD model described the adult data well (Fig. 2) and provided a statistically better fit to the current adult data set than the published model (drop in the objective function value of 16.2 with only one additional parameter). Changes in population parameter estimates included a 10 % increase in EC50-values and a 1–3 % drop in unexplained inter-individual variability (a full comparison of the parameter estimates of the published and bridged KPD model is provided in Table 2, Online Resource 1).
Fig. 2.
Prediction corrected VPC for the bridged KPD model applied on data from warfarin-treated adults. The solid line denote the median of observed data (circles) and dotted lines denote the 2.5th and 97.5th percentiles of observed data. Shaded areas represent 95 % confidence intervals of simulated 95 % prediction intervals and medians
Table 2.
Patient characteristics of paediatric patients included in I) evaluation of predictive performance of bridged KPD model and II) comparison of warfarin dosing algorithms for children
| Data I, N = 64 | Data II, N = 49 | |
|---|---|---|
| Sex (% male/female) | 52/48 | 53/47 |
| Median Age, years (range) | 4.3¶(0.055–18.9) | 7.2 (0.33–16.9) |
| Median Weight, kg (range) | 17¶(3.4–94) | 21.3 (5.2–61.1) |
| Median Height, cm (range) | 102¶(54–189.2) | 118 (63.7–185) |
| CYP2C9 genotype†, N (%) | ||
| *1/*1 | 47 (73.4) | 34 (69.4) |
| *1/*2 | 8 (12.5) | 7 (14.3) |
| *1/*3 | 7 (10.9) | 6 (12.2) |
| *2/*2 | 1 (1.6) | 1 (2.0) |
| *2/*3 | 1 (1.6) | 1 (2.0) |
| *3/*3 | – | – |
| VKORC1 genotype, N (%) | ||
| G/G | 24 (37.5) | 19 (38.8) |
| A/G | 31 (48.4) | 23 (46.9) |
| A/A | 9 (14.1) | 7 (14.3) |
| Treatment Indication, N (%) | ||
| Fontan procedure | 20 (31.2) | 13 (26.5) |
| Prosthetic heart valve (mitral or aortic) | 23 (35.9) | 20 (40.8) |
| Dilated cardiomyopathy | 8 (12.5) | 7 (14.3) |
| Other | 13 (20.3) | 9 (18.4) |
| Target INR, N (%) | ||
| 2.0–3.0 | 33 (54.6) | 28 (57.1) |
| 2.5–3.5 | 20 (31.2) | 16 (32.7) |
| Other (lower range < 2 or upper range > 3.5) | 8 (12.5) | 5 (10.2) |
| Missing | 3 (4.7) | 0 |
¶ At time of first dose/INR observation
† Genotyping for CYP2C9 variant alleles also included *5, *6 and *11 but none of these were found in the studied population
Paediatric patient characteristics
A total of 67 warfarin-treated Swedish children were enrolled between November 2010 and December 2011. Three children were excluded from analyses due to missing treatment histories. Treatment data for the 64 remaining children varied in length from 3 weeks up to 10 years, and together comprised >45,000 treatment days, and a total of 4,944 INR observations. Details on patient demographics, treatment indication and target INR are provided as Data I in Table 2. The mean age at first data record was 4.3 years, with a mean body weight of 17 kg. Age and weight ranges for the entire study period were 0.05–18.9 years and 3.4–94 kg, respectively. Self-reported information on ethnicity included 53 Caucasian, six Asian, two African and three with other or mixed ethnicity. The distribution of VKORC1 and CYP2C9 genotypes are provided in Table 2. Allele frequencies were as expected and no deviation from the Hardy-Weinberg equilibrium was observed. No patient carried the CYP2C9 *5, *6 or *11 variant alleles. A wide range of doses was administered in the study, ranging from 0.125 mg to 12.5 mg, or 0.022 mg/kg to 0.345 mg/kg. Most doses were administered as the marketed 2.5 mg tablet, but approximately 1 % used an extemporaneous capsule formulation. INR was mainly measured with Owren-based methods, with less than 13 % measured with a Quick-based self-monitoring system.
Evaluation of the bridged KPD model in children
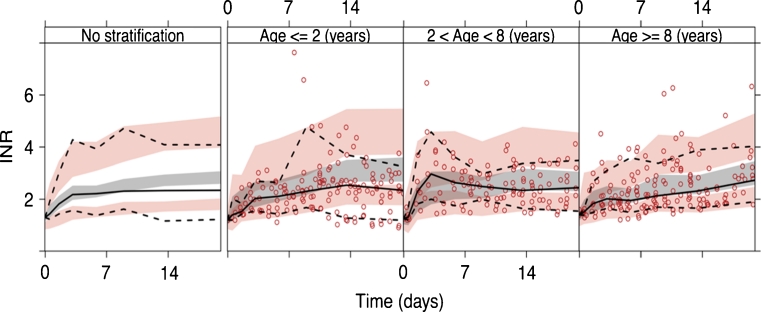
Figure 3 shows pc-VPCs for evaluation of predictive performance of the bridged KPD model in the 64 warfarin-treated children. Overall, the model performed well in predicting the INR response in children 0–18 years old. When stratified into three age groups (≤ 2 years, > 2 and < 8 years, and ≥ 8 years), there was a trend that the model overpredicted the INR response in the youngest age group.
Fig. 3.
Prediction corrected VPCs for the bridged KPD model applied on data from warfarin-treated children. The panel to the left represent data from all children (n = 64) and the three panels to the right are after stratification of data into three age groups; ≤ 2 years, > 2 and <8 years and ≥ 8 years. Solid lines denote the medians of observed data (circles) and dotted lines denote the 5th and 95th percentiles of observed data. Shaded areas represent 95 % confidence intervals of simulated 90 % prediction intervals and medians
Comparison of dose prediction accuracy
Forty-nine of 64 children fulfilled criteria for stable warfarin therapy. Data from the first stable treatment period (when several periods were available) were used to compare dose prediction accuracy between the different dose algorithms. Patient characteristics for this subset are included as Data II in Table 2. The median observed daily maintenance dose in this subset was 2.95 mg or 0.131 mg/kg, ranging from 0.56 mg to 9.11 mg or 0.036 mg/kg to 0.291 mg/kg.
Results on bias, imprecision and percentage of children with the maintenance dose underestimated (prediction <80 % of actual dose), ideal (prediction ± 20 % of actual dose) or overestimated (prediction >120 % of actual dose) are presented in Table 3. Bias ranged from −0.26 mg to 1.94 mg, and imprecision from 0.90 mg to 2.19 mg. The percentage of children with an ideal dose prediction was 33 % with an empiric 0.2 mg/kg dose, 35 % with the algorithm by Nowak-Göttl et al. [12], 33 % with Moreau et al. [13], and 41 % with Biss et al. [14] and the bridged KPD model. When information on previous doses and up to three INRs was used for a posteriori dose prediction with the bridged KPD model, the percentage of children with an ideal dose prediction increased to 70 %.
Table 3.
Percentage of children with predicted maintenance doses underestimated (prediction <80 % of actual dose), ideal (prediction ± 20 % of actual dose) or overestimated (prediction >120 % of actual dose), and bias and imprecision for the different dose prediction models
| Dose Underestimated | Dose Ideal | Dose Overestimated | Bias (Mean Prediction Error) | Imprecision (Root Mean Square Error) | |
|---|---|---|---|---|---|
| A priori dose predictions | |||||
| Fixed dose 0.2 mg/kg | 8 % | 33 % | 59 % | 1.94 | 2.19 |
| Algorithm by Nowak-Göttl et al. [12] | 28 % | 35 % | 37 % | −0.26 | 1.05 |
| Algorithm by Moreau et al. [13] | 16 % | 33 % | 51 % | 0.32 | 0.98 |
| Algorithm by Biss et al. [14] | 31 % | 41 % | 28 % | −0.03 | 0.90 |
| Bridged KPD model | 35 % | 41 % | 24 % | −0.13 | 0.94 |
| A posteriori dose prediction | |||||
| Bridged KPD model | 14 % | 70 % | 16 % | −0.04 | 0.57 |
Prediction errors for individual children and dose algorithms are shown in Fig. 4. Individual prediction errors ranged from a 400 % overprediction with the fixed 2 mg/kg dose regimen, to a 75 % underprediction with the algorithm by Moreau et al. [13]. For one of the children, the dose was overpredicted, with more than 150 % with all models except the algorithm by Nowak-Göttl et al. [12]. If this child is disregarded, the bridged KPD model showed the most narrow prediction error interval for a priori doses, ranging from −65 % to 59 %. Same type of plots but for children ≤2 years old (n = 11) or stratified on number of variant alleles, are presented as Figs. 3 and 4, Online Resource 1. Overall, the bridged KPD model and the algorithm by Biss et al. [14] showed the most narrow ranges of predictions errors.
Fig. 4.
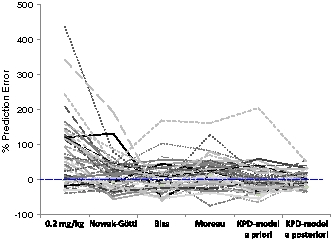
Percent prediction error in warfarin maintenance dose in children 0–18 years old. Results are provided as % prediction error in a priori predicted maintenance dose for one non-pharmacogenetic (empiric 0.2 mg/kg dose) and four pharmacogenetics-based algorithms, and in a posteriori predicted maintenance dose for the bridged KPD model. Results from the different models included in the comparison are connected for each child. Results above zero means that the dose was overpredicted and results below zero that the dose was underpredicted
Additional results, including details about individual predictors for all children included in the dose comparison, are provided in Fig. 5 and Table 3, Online Resource 1.
Discussion
In the present paper, a novel method for the prediction of warfarin dose in children is presented and compared with three published pharmacogenetics-based algorithms for maintenance dose prediction. The new method is based on a pharmacometric model that describes the complete time course of INR response to warfarin treatment. The model was derived from adult patient data and bridged to children based on commonly used allometric principles and a published maturation function for CYP2C9. In other words, no data or information from warfarin-treated children was used to develop the model for dose prediction in children.
Overall, the bridged KPD model predicts the INR response in children well, although there is a tendency that the model overpredicts the response, or conversely underpredicts the dose, in the youngest age group (≤ 2 years). This may be due to inaccurate prediction of the PK in children, i.e. that the bridging underestimates the metabolic capacity in this age group. However, this is likely not due to using post-natal age instead of post-menstrual age, in which case the differences between observations and predictions should have gone in the other directions. Online Resource 1 provides further discussion about the maturation function. The discrepancies could also be due to PD differences, for example that young children are less sensitive to warfarin than older children and adults. Another possibility is that it is caused by practical aspects of administering warfarin to young children, such as the crushing of tablets and mixing with food or liquids, which makes accurate and reproducible dosing difficult [2], and which could overestimate the actual dose delivered. In principle, predictions could be improved by updating the model and/or re-estimating the model parameters on data from warfarin-treated children. However, the currently available data set, with data from only 64 children between 0.06 and 18 years old and with only two children with two CYP2C9 variant alleles, was judged insufficient for doing this in a reliable and generalizable way for all ages and genotype combinations. Despite the overprediction of INR noted in the youngest age group with the bridged KPD model, it predicted daily maintenance doses as well or better than published dosing algorithms for children, although with a tendency towards underpredicting dose requirements.
When comparing the different dosing algorithms, it was clear that a fixed dose of 0.2 mg/kg would overestimate the daily maintenance dose in the majority of children. Admittedly, this comparison is somewhat unfair, since this was intended as a starting dose rather than a daily maintenance dose recommendation. However, it serves to illustrate the fact that a fixed dose regimen will not succeed in predicting the dose requirement across all patients. The other algorithms, including the bridged KPD model, with a more individualized approach, performed better in predicting the daily maintenance dose. One interesting observation is that the KPD model and the algorithm by Biss et al. [14] appeared to perform better than the other two, especially in children with at least one variant allele of CYP2C9 and/or VKORC1. These two methods both take the number of CYP2C9 and VKORC1 variant alleles into account, and also distinguish between the effect of CYP2C9*2 and *3.
A practical limitation with the bridged KPD model is that it is not easy to use compared to the other algorithms. They all use a simple equation to predict maintenance dose for a given child, while the bridged KPD model is based on a set of differential equations, without a closed form solution. To address this, work is currently ongoing to convert the NONMEM-model into a user-friendly tool for a priori and a posteriori dose predictions in both children and adults.
An advantage with the bridged KPD model is that it describes the whole time course of treatment, and can be used for both a priori and a posteriori dose predictions. In the example presented, the percentage of children with the dose ideally predicted (± 20 % of actual dose) increased from 41 % with a priori prediction to 70 % with a posteriori revision of warfarin maintenance dose. Besides dose predictions, the model can also be used for prediction of early INR response for a given dose regimen, which is not possible with the other algorithms. This could help to identify situations where warfarin therapy could be improved with loading doses and/or delayed dose-adjustments.
In conclusion, the bridged KPD model performed at least as well for warfarin maintenance dose prediction in children as algorithms developed on data from children. Since it is possible to further refine the bridged KPD model with paediatric data, it is a promising first step towards a more individualised warfarin therapy in children.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
A-K Hamberg is supported by a personal grant from the Ränk family via the Swedish Heart and Lung Foundation. Grants from the Swedish Research Council (Medicine 523-2008-5568), the Swedish Heart and Lung Foundation, the Clinical Research Support (ALF) at Uppsala University, and the Swedish Academy of Pharmaceutical Sciences supported the performance of this study. The authors acknowledge the following persons for study co-ordination: A Johansson Research Nurse (RN) at The Queen Silvia Children’s Hospital, Sahlgrenska University Hospital, Gothenburg; A Maxedius RN at Children's Heart Center, Skåne University Hospital, Lund; Å Burström RN at Astrid Lindgren Children’s Hospital, Stockholm; and E Mattsson RN at Uppsala University Children´s Hospital, Uppsala. A Öhman, MD at Astrid Lindgren Children’s Hospital, Stockholm, is also acknowledged for facilitating data collection. The authors are grateful to H Kohnke for performing the genotyping. We also wish to thank all the children that participated in this study and their parents.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, Vesely SK. Antithrombotic Therapy in Neonates and Children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e737S–e801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monagle P, Newall F, Campbell J. Anticoagulation in neonates and children: Pitfalls and dilemmas. Blood Rev. 2010;24(4–5):151–162. doi: 10.1016/j.blre.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Young G. Old and new antithrombotic drugs in neonates and infants. Semin Fetal Neonatal Med. 2011;16(6):349–354. doi: 10.1016/j.siny.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Wadelius M, Chen LY, Lindh JD, Eriksson N, Ghori MJR, Bumpstead S, Holm L, McGinnis R, Rane A, Deloukas P. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2009;113(4):784–792. doi: 10.1182/blood-2008-04-149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gage B, Eby C, Johnson J, et al. Use of Pharmacogenetic and Clinical Factors to Predict the Therapeutic Dose of Warfarin. Clin Pharmacol Ther. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Warfarin Pharmacogenetics Consortium, Klein TE, Altman RB et al Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360(8):753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenzini P, Wadelius M, Kimmel S, et al. Integration of Genetic, Clinical, and INR Data to Refine Warfarin Dosing. Clin Pharmacol Ther. 2010;87(5):572–578. doi: 10.1038/clpt.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avery PJ, Jorgensen A, Hamberg A-K, Wadelius M, Pirmohamed M, Kamali F. A Proposal for an Individualized Pharmacogenetics-Based Warfarin Initiation Dose Regimen for Patients Commencing Anticoagulation Therapy. Clin Pharmacol Ther. 2011;90(5):701–706. doi: 10.1038/clpt.2011.186. [DOI] [PubMed] [Google Scholar]
- 9.van Schie RM, Wadelius M, Kamali F, et al. Genotype-guided dosing of coumarin derivatives: the European pharmacogenetics of anticoagulant therapy (EU-PACT) trial design. Pharmacogenomics. 2009;10(10):1687–1695. doi: 10.2217/pgs.09.125. [DOI] [PubMed] [Google Scholar]
- 10.French B, Joo J, Geller NL, Kimmel SE, Rosenberg Y, Anderson JL, Gage BF, Johnson JA, Ellenberg JH, COAG Investigators (2010) Statistical design of personalized medicine interventions: The Clarification of Optimal Anticoagulation through Genetics (COAG) trial. Trials 1–9 [DOI] [PMC free article] [PubMed]
- 11.Do EJ, Lenzini P, EBY CS, et al. (2011) Genetics informatics trial (GIFT) of warfarin to prevent deep vein thrombosis (DVT): rationale and study design. Pharmacogenomics J 1–8 [DOI] [PMC free article] [PubMed]
- 12.Nowak-Gottl U, Dietrich K, Schaffranek D, Eldin NS, Yasui Y, Geisen C, Mitchell LG. In pediatric patients, age has more impact on dosing of vitamin K antagonists than VKORC1 or CYP2C9 genotypes. Blood. 2010;116(26):6101–6105. doi: 10.1182/blood-2010-05-283861. [DOI] [PubMed] [Google Scholar]
- 13.Moreau C, Bajolle F, Siguret V, Lasne D, Golmard JL, Elie C, Beaune P, Cheurfi R, Bonnet D, Loriot MA. Vitamin K antagonists in children with heart disease: height and VKORC1 genotype are the main determinants of the warfarin dose requirement. Blood. 2012;119(3):861–867. doi: 10.1182/blood-2011-07-365502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biss TT, Avery PJ, Brandao LR, Chalmers EA, Williams MD, Grainger JD, Leathart JBS, Hanley JP, Daly AK, Kamali F. VKORC1 and CYP2C9 genotype and patient characteristics explain a large proportion of the variability in warfarin dose requirement among children. Blood. 2012;119(3):868–873. doi: 10.1182/blood-2011-08-372722. [DOI] [PubMed] [Google Scholar]
- 15.Holford N. Dosing in children. Clin Pharmacol Ther. 2010;87(3):367–370. doi: 10.1038/clpt.2009.262. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi H. Developmental changes in pharmacokinetics and pharmacodynamics of warfarin enantiomers in Japanese children. Clin Pharmacol Ther. 2000;68(5):541–555. doi: 10.1067/mcp.2000.110977. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Ichida F, Saito K, Watanabe K, Hirono K, Miyawaki T, Yoshimura N, Horiuchi I, Taguchi M, Hashimoto Y. Effect of the VKORC1 Genotype on Warfarin Dose Requirements in Japanese Pediatric Patients. DMPK. 2011;26(3):295–299. doi: 10.2133/dmpk.DMPK-10-NT-082. [DOI] [PubMed] [Google Scholar]
- 18.Meibohm B, Läer S, Panetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7(2):E475–87. doi: 10.1208/aapsj070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edginton AN. Knowledge-driven approaches for the guidance of first-in-children dosing. Pediatric Anesthesia. 2010;21(3):206–213. doi: 10.1111/j.1460-9592.2010.03473.x. [DOI] [PubMed] [Google Scholar]
- 20.Cella M, Gorter de Vries F, Burger D, Danhof M, Pasqua Della O. A model-based approach to dose selection in early pediatric development. Clin Pharmacol Ther. 2010;87(3):294–302. doi: 10.1038/clpt.2009.234. [DOI] [PubMed] [Google Scholar]
- 21.Hamberg A-K, Dahl ML, Barban M, Scordo MG, Wadelius M, Pengo V, Padrini R, Jonsson EN. A PK-PD model for predicting the impact of age, CYP2C9, and VKORC1 genotype on individualization of warfarin therapy. Clin Pharmacol Ther. 2007;81(4):529–538. doi: 10.1038/sj.clpt.6100084. [DOI] [PubMed] [Google Scholar]
- 22.Hamberg A-K, Wadelius M, Lindh JD, Dahl ML, Padrini R, Deloukas P, Rane A, Jonsson EN. A pharmacometric model describing the relationship between warfarin dose and INR response with respect to variations in CYP2C9, VKORC1, and age. Clin Pharmacol Ther. 2010;87(6):727–734. doi: 10.1038/clpt.2010.37. [DOI] [PubMed] [Google Scholar]
- 23.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-Corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. AAPS J. 2011;13(2):143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer RJ. NONMEM User´s Guide. Ellicott City: Icon Development Solutions; 2011. pp. 1–128. [Google Scholar]
- 25.Jönsson S, Karlsson MO. Estimation of dosing strategies aiming at maximizing utility or responder probability, using oxybutynin as an example drug. Eur J Pharm Sci. 2005;25(1):123–132. doi: 10.1016/j.ejps.2005.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.