Abstract
Dithiocarbamates are a class of metal-chelating compounds with various applications in medicine. They have been used for the treatment of bacterial and fungal infections, possible treatment of AIDS, and most recently cancer. Their anti-tumor effects can in part be attributed to their ability to complex tumor cellular copper, leading to binding to and inhibition of the proteasome and in turn initiating tumor cell-specific apoptosis. Current chemotherapeutic agents are highly toxic and therefore their efficacy in the eradication of tumors is greatly limited. As a result many scientists have joined the quest for novel targeted therapies in hopes of reducing toxicity while maximizing potency and proteasome inhibition has become an attractive therapy in this regard. Here we discuss the origins, mechanism, and evolution of dithiocarbamates as potent proteasome inhibitors and therefore anti-cancer agents.
Keywords: Cancer therapy, Ubiquitin-Proteasome System, Apoptosis, Metal Complexes, Disulfiram, Antabuse, Proteasome inhibitors, UPS inhibitors
INTRODUCTION
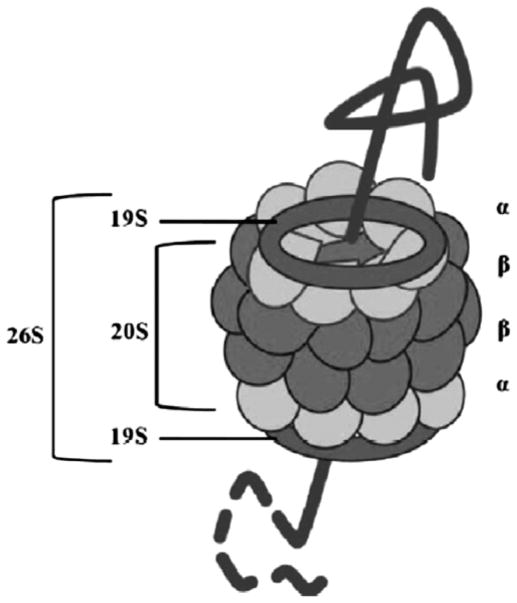
The ubiquitin proteasome system (UP-S) (Fig. 1) functions as the cell’s housekeeping service in terms of protein turnover; tightly regulating the delicate balance between the production of new proteins and the targeted degradation of others. Its function is essential not only in maintaining protein homeostasis, but also in ensuring that critical cellular regulatory processes like transcription, DNA damage and repair, cell cycle, apoptosis and protein trafficking take place as needed. In fact, previous studies have elucidated the role of the UP-S in the degradation of more than 80% of cellular proteins [1–3]. Furthermore, its hierarchical nature provides a rich source of molecular targets for specific intervention and it has therefore arisen as a promising approach to innovative anticancer therapies [1–3].
Fig. 1.
The 26S proteasome. The unfolded protein destined for degradation by the proteasome is recognized by the 19S regulatory subunit. As it proceeds through the 20S catalytic core, made up of two outer α rings and the two inner β rings, it is proteolytically chopped up into amino acid subunits which are recycled in the cytosol.
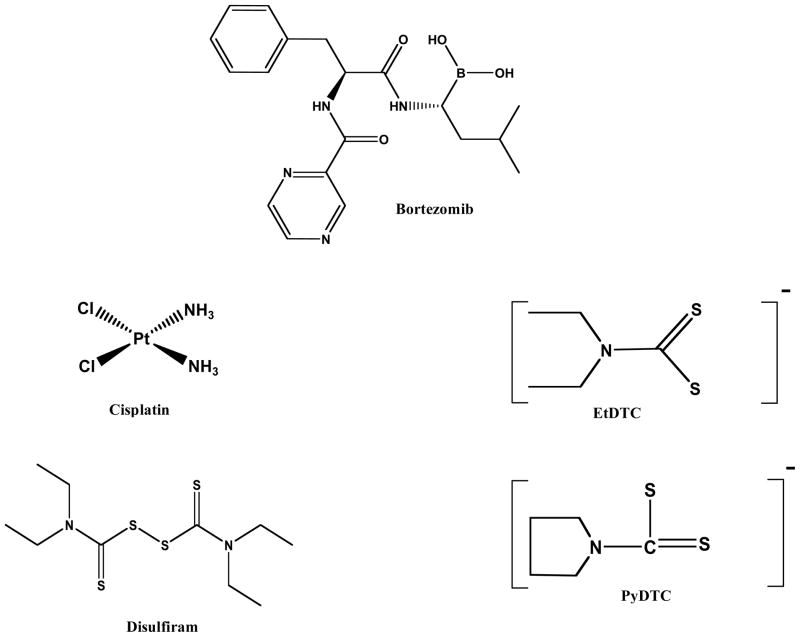
The first contender in this pathway being investigated as a target for cancer therapy is the proteasome. The use of proteasome inhibitors triggers a mixed repertoire of tumor-suppressing and prosurvival pathways in cancer cells, disturbing the critical intracellular balance of the proteins in these pathways, thus shifting signals towards apoptosis and tumor inhibition [4, 5]. Bortezomib (Fig. 2), an already FDA approved highly selective proteasome inhibitor, has been evaluated in the clinic in combination chemotherapy and as a single agent for the treatment of relapsed multiple myeloma and has received much positive feedback. Although successful in improving clinical outcomes when used in hematological malignancies, relapse often occurs in those patients who responded initially [4, 5]. Furthermore, some adverse side effects have been reported with its clinical use including myelosuppression, thrombocytopenia, gastrointestinal effects, shingles, and peripheral neuropathy in 30% of patients. The use of bortezomib in other cancers including solid tumors has also been investigated in clinical studies. It was found that bortezomib has shown little promise in solid tumors [4, 5]. The combination of its reported side effects in patients, development of drug resistance, and lack of clinical benefit in solid tumors has made way for the discovery of other novel proteasome inhibitors, such as second generation proteasome inhibitors (such as carfilzomib and marizomib) and other agents with proteasome-inhibitory function such as dithiocarbamates [6]. The dithiocarbamate class of metal-complexing compounds has become a novel emerging class of anticancer proteasome inhibitors with great potential to overcome the limitations seen with bortezomib use [3, 6].
Fig. 2.
Chemical structures of bortezomib, cisplatin and several dithiocarbamate compounds.
THE PROTEASOME
Proteasomes are localized in the nucleus and cytosol and can constitute up to 1% of the cellular protein content in eukaryotes [7, 8]. The 26S proteasome was first described in 1988 as a large multi-subunit complex assembled from ring-shaped 19S regulatory and 20S catalytic core components, which are composed of numerous polypeptide subunits (Fig. 1) [7, 9, 10]. The 20S catalytic core can best be described as a cylindrical stack of 4 rings forming a narrow pore: two outer rings each containing 7α subunits and two inner rings containing 7β subunits each [7]. Furthermore, each subunit in this collection has a distinct function. For example, β2 and β5 subunits grant trypsin-like and chymotrypsin (CT)-like activities to the proteasome, while the β1 subunit is characterized as having caspase-like (peptidylglutamyl-peptide hydrolyzing/PGPH) effects, with the ability to cleave bonds on the carboxyl side of basic, hydrophobic, or acidic amino acid residues, respectively [7, 11]. Therefore, the degradation of proteins inside the proteasome is certainly comparable to the degradation of proteins by intestinal digestive enzymes where proteins are also broken down in several steps.
The proteasome is highly selective for the breakdown of ubiquitin-tagged target proteins; involving a multi-step process which requires ATP and is regulated by three important enzyme types: E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzymes, and the E3 ubiquitin ligases. It is the polyubiquitination of particular protein substrates that directs them for degradation by the proteasome. At the proteasomal core, allosteric interactions guide the intricate sequencing of proteolytic reactions, which ultimately results in the production of oligopeptides that can be recycled within the cell [12]. Given its crucial role in the cell, it was important to determine if an activity differential exists between the normal and diseased cellular proteasome in order to target it therapeutically, and in fact when the activity level of the 20S catalytic core was investigated in healthy individuals versus those having various neoplastic conditions, the results showed that the proteasome catalytic core was markedly increased (1000-fold) in the plasma of patients in a pathological state [13]. Moreover, many cancer-associated proteins such as p53, p27, epidermal growth factor receptor (EGFR), and transforming factor-β receptor (TGF-βR) are regulated by the proteasome, attesting to its importance in the maintenance of cellular regulatory mechanisms [14–17]. Stabilization and/or destabilization of such proteins disrupts protein homeostasis within the cell, leading to abrogated cell signaling pathways and thus a chaotic state for the cell we call cancer, making the proteasome an excellent target for therapeutic intervention in the treatment of this group of diseases.
DITHIOCARBAMATES AND METALS AS ANTICANCER DRUGS
Metal complexes possess unique reactivity states and provide scientists with a broad spectrum of possibilities in terms of synthesizing novel compounds with antitumor potential. Indeed, the wide range of coordination numbers and geometries, accessible redox states, thermodynamic and kinetic characteristics, and the intrinsic properties of both the cationic metal ion and ligand itself offer the medicinal chemist a wide array of reactivity that can be exploited [18]. Furthermore, the transition metals provide a rich palette from which to choose and offer a real possibility for the discovery of truly novel drugs with new mechanisms of action [18, 19]. For example, a variety of ruthenium complexes have been designed which interact specifically with the classical metallo-drug target: DNA [20, 21]. A family of ruthenium(II)-arene complexes developed by Sadler et al. [22] for instance, exhibit high in vitro and in vivo anticancer activity. However, aside from the cisplatin-mediated DNA targeting proto-type mechanism, the metallodrug-protein interactions are a new area of research with much potential in cancer therapy [20]. Studies involving these types of interactions will not only aid in identifying the mechanism of action of a particular metal complex, but can also identify novel protein therapeutic targets [20].
Dithiocarbamates in particular have the ability to modulate key proteins involved in biological processes including apoptosis, oxidative stress, transcription and degradation, which has generated interest in these compounds as not only anticancer agents but for treatment of an assortment of other conditions including cocaine addiction [23], inflammation [24], and viral infections [25]. Their use as proteasome inhibitors in the treatment of cancer was initially frowned upon as the UP-S is responsible for protein maintenance in normal functioning cells and therefore this target was deemed non-specific. Not until cancer cells, specifically, were shown to be much more responsive to proteasome inhibition than normal cells did this concept become more widely accepted [26–28]. However, inhibition of the proteasome’s chymotrypsin-like (CT-like) activity, and not the other activities exhibited by the proteasome, is most effective in inducing cancer cell death by apoptosis [29]. Therefore, it is desirable to synthesize dithiocarbamates specific for the chymotrypsin β5 site and this is currently a very active field in cancer research. Metal complexing agents currently at the forefront include copper, gold and zinc, all of which have shown to be more specific for the chymotrypsin-like site over other sites of the proteasome [30, 31].
CISPLATIN, A PLATINUM CONTAINING ANTI-CANCER DRUG
The use of metals in medicine dates back to the early days of human civilization, and the evolution of this concept has led to the discovery of cisplatin (Fig. 2), the cytotoxic platinum-containing agent that is now a widely used chemotherapeutic drug. The first of its kind, today cisplatin is one of the most effective chemotherapy agents in the treatment of ovarian, small-cell lung, bladder, cervical, brain, lung, and breast cancers. Despite its therapeutic success, cisplatin has several major adverse side effects which limit its clinical use, the most alarming being nephrotoxicity. Less toxic variations of cisplatin have produced carboplatin and oxaliplatin, along with second and third generation platinum compounds, including trans-Pt(II)-imini derivatives and polynuclear platinum(II) complexes [32, 33]. Furthermore, second and third generation compounds with alternative amino and/or anionic ligands have also been synthesized [34]. Especially favorable in this group, due to the convenience of not having to hospitalize patients to administer it, is the oral JM216/satraplatin, a platinum(IV) complex which is reduced in vivo to platinum(II) [34]. Although, its mechanism of action involves irreversibly crosslinking DNA and ultimately triggering apoptosis, rather than specifically targeting the proteasome, the discussion at hand, cisplatin has set the foundation for the use of metal complexing agents in cancer therapy. Since its discovery in the 1960s and FDA approval in 1978, several variations have been derived and the wide success of platinum drugs has promoted the development of both platinum derivatives and other metal based compounds [35]. However, the latest approaches in anti-cancer metal based therapeutics have shifted their focus to non-platinum agents because of the severe toxicity commonly associated with cisplatin and platinum-based compounds. Of the potential non-platinum drugs, gold complexes have recently gained an increasing amount of attention due to their strong tumor cell growth inhibiting effects generally achieved by exploiting non-cisplatin-like pharmacodynamics and pharmacokinetic properties and mechanisms of action [36, 37].
GOLD DITHIOCARBAMATES AS TUMOR PROTEASOME INHIBITORS
The earliest therapeutic application of gold can be tracked back to 2500 BC in China where gold was used to treat smallpox, skin ulcers, and measles [38]. During this time people viewed gold as a symbol of immortality and commonly associated it with longevity, with some cultures still believing in the healing powers of gold today. Remarkably so, these beliefs and ideas have withstood and evolved to present day, where gold compounds are being investigated as potential candidates in the fight against cancer. Gold (I) complexes, including auranofin (Ridaura) analogs, have been synthesized and found to be potent against B16 melanoma and P388 leukemia cells [39]. Of the gold (I) compounds synthesized, the phosphine-gold (I) thiosugars were most potent against leukemia in vivo, however, these analogs were completely inactive against solid tumors [40]. Similarly, digold (I) phosphine complexes were also found to be inactive against solid tumors and did not enter clinical trials due to their severe cardiotoxicity [41]. Although their use as anticancer drugs was originally questioned due to high redox activity and poor stability, gold (III) complexes have also been investigated. Interest in these complexes increased after Pt (II) complexes showed positive results, due to the fact that Au (III) and Pt (II) are isoelectronic and tetracoordinate gold (III) complexes share the same square planar geometry as cisplatin [42]. Recently, various gold (III) compounds have been synthesized using ligand platforms containing nitrogen atoms as donor groups [43]. These newly renovated compounds exhibit a superior chemotherapeutic index to cisplatin due to greater bioavailability, increased potency, and fewer overall toxic side effects [44].
Gold diothiocarbamates, particularly derivatives of N,N-dimethyldithiocarbamate and ethylsarcosinedithiocarbamate, such as Au (DMDT)Cl2, Au (DMDT)Br2, Au (ESDT)Cl2 and Au (ESDT)Br2, have been evaluated for their potential to inhibit cisplatin-induced nephrotoxicity and for in vitro cytotoxicity toward a variety of human tumor cell lines [45, 46]. They demonstrated a 1–4 fold increase in potency compared to cisplatin and were able to overcome intrinsic and acquired cisplatin resistance [44]. These dithiocarbamates are fast-acting in their ability to inhibit RNA and DNA synthesis and show only minimal cross-resistance with cisplatin, suggesting a different mechanism of action [42].
Recent evidence suggests that the cellular proteasome is a molecular target for gold complexes and the mechanism of action underlying their activity is only beginning to emerge. In an attempt to discern a possible mechanism of action, we selected Au (DMDT)Br2 and tested its proteasome-inhibitory potential. We reported that the CT-like activity of the purified 20S proteasome (IC50= 7.4 μM) and 26S proteasome in intact MDA-MB-231 breast cancer cells (10–20 μM) was significantly inhibited by Au (DMDT)Br2. PGPH-like and trypsin-like activities were also inhibited, but the CT-like inhibition was the most significant, indicating that this complex preferentially binds to and inhibits the CT-like β5 subunit of the proteasome. Associated with proteasomal inhibition, an accumulation of ubiquitinated proteins and p27, as well as induction of apoptosis were observed in these breast cancer cells. Additionally, Au (DMDT)Br2 was able to potently inhibit tumor growth (~50%), associated with inhibition of proteasomal CT-like activity (40%) in breast cancer xenografts [30].
We have also investigated the effect of two gold compounds with different oxidation states toward the cellular proteasome, and endeavored to gain insight into their potential mechanism of action. We compared the effects of two gold compounds, gold (I) compound (Au (ESDT)2), AUL15, to a gold (III) compound (AuBr2 (ESDT)), AUL12, on breast cancer cells and the proteasome. The results showed that while both complexes inhibited the growth of MDA-MB-231 breast cancer cells, AUL12 was much more potent (IC50= 4.5 μM, 70% inhibition) than AUL15 (IC50= 13.5 μM, 35% inhibition). We also observed that both complexes were able to inhibit purified 20S proteasome (AUL12 IC50= 1.13 μM; AUL15 IC50= 17.7 μM) as well as intact 26S proteasome, again with AUL12 exhibiting much higher activity [47]. Additionally, we observed that AUL15 inhibits the cellular proteasome much later (> 24 hr) compared to AUL12 (4 hr) in intact breast cancer cells. Associated with these effects was the accumulation of ubiquitinated proteins and IκB-α as well as induction of cell death as demonstrated by PARP cleavage and increased levels of Bax dimers. These death-associated changes appeared much later in AUL15-treated cells compared to the AUL12-treated cells. In an effort to gain insight into the mechanism of action responsible for their biological effects, we investigated whether these gold compounds could induce the production of reactive oxygen species. Interestingly, we found that treatment with AUL12 (Au (III)), but not AUL15 (Au (I)), was associated with redox processes, suggesting that induction of oxidative stress may be partially responsible for the cytotoxic activity of gold (III) compounds [47]. Thus, metals other than platinum show promise as anti-cancer agents, specifically as proteasome inhibitors, and not surprisingly, other metals have also been investigated.
COPPER AND ZINC DITHIOCARBAMATES AS TUMOR PROTEASOME INHIBITORS
Copper and zinc are not only indispensable metals involved in many critical biological processes like respiration, protein modifications, and angiogenesis - these metals have also gained considerable interest as potential anticancer drug targets. Given their important physiological functions, it is not surprising that concentrations of both zinc and copper are tightly regulated. Nevertheless, disturbed zinc homeostasis and elevated copper levels have been reported in many cancer types, including breast, prostate, lung and brain tumors [37, 48]. In 1980, it was first noticed that Cu played a critical role in angiogenesis [49]. Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis and can stimulate growth, migration and differentiation of endothelial cells from existing blood vessels; cell culture and animal studies have demonstrated that Cu is able to induce VEGF transcription and protein expression [50, 51]. Due to the importance of angiogenesis and copper to tumor development, the use of copper chelators for antiangiogenic therapy in addition to proteasome inhibition has emerged as an interesting concept in cancer therapeutics [52, 53].
Although Zn levels are often compromised in cancer patients, a firm relationship between cancer development and Zn has yet to be proven, and seems dependent on tumor type [54–56]. Low levels of zinc have been observed in several malignancies, such as those of the liver, gallbladder, digestive tract, and prostate [57–59]. Conversely, both high and low levels of zinc have been found in breast cancers [57, 60, 61]. Zinc is a structural component of various proteins and enzymes such as transcription factors, cell signaling proteins, and DNA repair enzymes and it is thought to have a critical role in apoptosis [54, 62–65]. However, this effect appears to be complex and specific to tumor type so that no firm conclusions have been established. For example, in prostate and ovarian epithelial, as well as glial cells, zinc is pro-apoptotic, while in breast, HeLa, renal, and lung epithelial cells, as well as macrophages, zinc is anti-apoptotic [54, 66].
We have examined the possible chemotherapeutic properties of pyrrolidinedithiocarbamates (PyDTC) dithiocarbamate complexes. When coupled with copper and zinc we found that both Zn(PyDTC) and Cu(PyDTC) complexes exhibited proteasome inhibitory activity against purified 20S proteasome as well as intact 26S proteasome in MDA-MB-231 cells [31]. Accumulation of ubiquitinated proteins and proteasomal target proteins IκB-α and p27 was observed, and apoptosis associated morphological changes as well as PARP cleavage occurred in cells treated with either Zn(PyDTC) or Cu(PyDTC). Furthermore, the effects of these PyDTC complexes were time-dependent, with >50% inhibition occurring at early time points [31]. To further examine the results observed with PyDTC complexes, we synthesized PyDTC:metal complexes in a 2:1 ratio (Zn (PyDTC)2 and Cu (PyDTC)2). We determined that these synthetic complexes were much less potent toward purified 20S proteasome (40% inhibition at 50 μM), but more potent toward intact 26S proteasome in MDA-MB-231 cells, with Cu (PyDTC)2 exhibiting higher activity than Zn (PyDTC)2. These synthetic complexes were also effective in other cell lines, including breast cancer DCIS and MCF7, and prostate cancer PC-3 cells [31]. Therefore, the use of metal-complexed dithiocarbamate species as proteasome inhibitors is a very promising strategy for the treatment of cancer.
Furthermore, through collaboration with Dr. B. Cvek, we synthesized a series of three complexes with diethyldithiocarbamate ligand and three different metals (Ni, Cu, Zn), confirmed by X-ray crystallography, and tested their effects in human breast cancer MDA-MB-231 cells. Zinc and copper complexes, but not the nickel complex, were found to be more active against cellular 26S proteasome than against purified 20S proteasome core particle. One of the possible explanations for this is inhibition of the JAMM domain in the 19S proteasome lid [67].
DISULFIRAM: AN “OLD” DRUG WITH A “NEW” PURPOSE
An exciting new concept is ongoing in the cancer research field that takes advantage of previously approved drugs, like metal chelators, used for the treatment of some other pathological conditions and advancing them for use as anti-cancer agents. Such drugs as disulfiram (DSF), diethyldithiocarbamate (EtDTC), and pyrrolidinedithiocarbamates (PyDTC) (Fig. 2) form metal complexes within cells and have been rigorously investigated as potential novel anticancer agents that target the UP-S. Disulfiram (tetraethylthiuram disulfide) has been on the market as Antabuse for decades and is one of only two drugs approved for the treatment of chronic alcoholism, owing to its ability to irreversibly inhibit aldehyde dehydrogenase (ALDH) and therefore induce an immediate hangover effect in the patient if alcohol is ingested. DSF is taken orally and is very tolerable, extremely potent, and has no toxicity associated with it, provided the patient abstains from drinking alcohol [68–70]. The structure of DSF contains an R1R2NC (S)SR3 functional group, with sulfhydryl groups that grant it the ability to react with Cu (II) (Fig. 2) [71]. This reaction can be confirmed by mixing DSF and CuCl2 at a 1:1 ratio and observing an intense color change [72]. Although DSF is not suitable for binding various other biological metal ions such as, Fe (II or III) or Mn (III) [71], it has been reported that DSF is also able to interact with Zn (II). In fact, Brar et al. reported that DSF treatment of melanoma and hepatic cancer could be potentiated by Zn (II) supplementation [73].
Our lab has demonstrated that the DSF-Cu complex is able to inhibit purified 20S proteasome (IC50=7.5 μM) and 26S proteasome in intact MDA-MB-231 breast cancer cells (20 μM). The CT-like activity was inhibited by >95% and proliferation was inhibited by up to 85% under the used experimental condition; importantly, normal breast MCF-10A cells exhibited no response to DSF, indicating a lack of toxicity, as well as a therapeutic strategy that utilizes heightened levels of copper as a tumor-targeting mechanism [72]. We have also reported the ability of DSF to inhibit the proteasome under in vivo conditions. Daily treatment of mice bearing MDA-MB-231 xenografts with 50 mg/kg DSF for 30 days resulted in significant tumor growth inhibition (74%). Associated with this growth inhibition, a significant decrease in proteasomal CT-like activity (87%) and accumulation of ubiquitinated proteins, p27, and Bax were visible. Furthermore, apoptosis-associated increases in caspase-3 activity and PARP cleavage were also observed [72].
The tumor suppressive effects of DSF often depend on passive cellular uptake in complexes with copper. Furthermore, copper ions were shown to be involved in regulation of dithiocarbamate-induced apoptosis of thymocytes, prostate cancer cells, astrocytes, and melanoma cells [71, 73–77]. Recently, the copper-mediated inhibition of histone acetyltransferase activity and induction of apoptosis in pyrrolidine dithiocarbamate-treated leukemic cells have also been reported [78]. Another potential target of DSF is superoxide dismutase, the inhibition of which may be associated with the inhibition of angiogenesis [79]. Furthermore, the known ability of DSF to inhibit aldehyde dehydrogenase (ALDH), the key enzyme involved in alcohol breakdown and recently identified as a breast cancer stem cell marker [80], is promising in the regard of preventing chemoresistance and cancer recurrence. Cancer stem cells are the major cause of chemotherapy failure and DSF has been shown to aid in their inhibition, thereby eliminating tumors at their so-called “root” [81]. DSF also potentiates the cytotoxicity of cyclophosphamide, cisplatin and radiation in vitro and protects normal cells in the kidney, gut and bone marrow in vivo, while increasing the therapeutic index of a wide range of cytotoxic drugs [82–84]. Others have identified DSF as a demethylating agent, RING-finger E3 ligase inhibitor, ROS-MAPK and NFκB modulator, only adding to its list of beneficial anti-cancer qualities [84–86].
The antitumor effects of DSF have been reported as early as the 1970s when Wattenberg published several studies showing that disulfiram, if added to the diet of mice, inhibited large bowel neoplasia and neoplasia in the forestomach produced by administration of the carcinogens dimethylhydrazine and benzo[α]pyrene, respectively [87, 88]. To date, ~200 publications are listed in the PubMed database harmonizing disulfiram with cancer and it is actively being studied in several clinical trials. Cancer is a disease of huge social and economic burden and disulfiram is an excellent example of a known drug that is readily available, cost effective, and has great potential for success in the treatment of this disease. Because it has been on the market for decades, information about any possible adverse side effects as well as documentation of safe concentrations in the human body are readily available [89]. Importantly, DSF in safe concentrations possesses anti-cancer effects. It is selectively cytotoxic to cells of chronic lymphoid leukemia, melanoma, and breast carcinomas; leaving non-cancer cells unaffected [72–74, 88]. Being in the midst of a financial crisis as we are, researchers and physicians alike should question old drugs like disulfiram for new answers. In addition, the government can contribute by supporting off-patent drug development in an effort to bring down the high costs of producing new drugs which sometimes only extend life by as little as a few weeks.
OTHER PROTEIN TARGETS OF DITHIOCARBOMATES
In addition to the ability of dithiocarbamates to inhibit the proteasome, several other mechanisms of action have been reported to be associated with their cell death-inducing ability. For instance, dithiocarbomates are among the most reported inhibitors of the nuclear factor-kappaB (NFκB) signaling cascade [90]. NFκB plays an important role in immune system function by controlling the expression of genes involved in the inflammatory response, cell adhesion, differentiation, oxidative stress and has been found to play a major role in many cancers through its protective effects against apoptosis. PyDTC stimulates intracellular zinc transport and thus subsequently modulates NFκB activity, stimulating cell death [90]. Studies of Lui G et al. also demonstrate that PyDTC blocks the activation of NFκB induced by paclitaxel resulting in increased sensitivity to paclitaxel and increased apoptosis in the case of ovarian cancer [91]. These data suggest that the combination of dithiocarbomates and paclitaxel can be used to overcome the risk of paclitaxel resistance [91]. In the case of colorectal cancers, PyDTC has been shown to inhibit NFκB activation thus suppressing tumor growth [92]. Studies have also shown PyDTC to have anti-proliferative and pro-apoptotic effects in prostate cancer [77], T-cell leukemia [93], gastric cancer, renal cell carcinoma [94; 95] and breast cancer cells [96].
Carbonic anhydrases (CA) are the zinc containing metalloenzymes that catalyze the hydration of carbon dioxide and encode at least thirteen enzymatically active isoforms with different structural and catalytic properties. In hypoxia the expression of CAs is altered, and CA IX, a transmembrane isoform, is overexpressed in hypoxic cancers, glaucoma and solid tumors [97]. In the recently published studies of Fabrizio Carta et al, dithiocarbomates were tested for their inhibitory activity on carbonic anhydrase isoforms and their SAR of action was reported by resolving x-ray crystal structures, showing that the organic scaffold of DTC is deeply buried in the active site forming a highly stable enzyme-inhibitor adduct [98]. Thus, these studies suggested the use of dithiocarbomates as ideal candidates for developing novel anti-glaucoma therapies targeting carbonic anhydrases.
Ubiquitin E3 ligases, which catalyze the final mechanistic step of ubiquitin conjugation to a target protein before proteasomal degradation, are known to be deregulated in cancer. One such E3 ligase is Breast Cancer Associated gene 2 (BCA2), isolated from an invasive breast cancer cell line [99]. BCA2 was shown to be highly expressed in invasive breast cancers and its down regulation inhibits breast cancer cell growth and invasiveness [99]. Studies conducted by Brahemi et al. suggest that dithio(peroxo)thioate compounds have potent activity at sub-micromolar concentrations in effectively inhibiting BCA2-expressing MCF7 and T47D breast cancer cell growth as well as the autoubiquitination activity of BCA2, a hallmark of all E3 ligases [86]. Thus, these compounds were suggested for use in inhibiting the action of zinc binding E3 ligases upstream of the proteasome in the ubiquitn-proteasome pathway.
Many tumors are under constant oxidative stress due to increased production of free radicals as a result of increased metabolic activity and decreased antioxidant levels. Therefore, the idea of targeting oxidative stress using antioxidants like PyDTC has been evaluated in breast cancer therapy. Studies by Jian-Wei Gu et al. in female mice bearing breast tumors suggest that PyDTC targets the expression of vascular endothelial growth factor (VEGF) expression and thus decreases angiogenesis [100]. Generally speaking, dithiocarbomates may thus be useful for targeting different biological pathways including the proteasome in cancer therapy.
CONCLUSION
The vital role the ubiquitin-proteasome system plays in the maintenance of protein homeostasis in all cells is essential for life and therefore components of this pathway were not considered “druggable” until recently. Cancer cells tend to have over-active proteasome activity and the development of bortezomib as a specific inhibitor represented a major advancement in this field, opening the door for the discovery of other proteasome-specific inhibitors in the hopes of uncovering agents that would be especially more potent in solid tumors. Furthermore, current research suggests that a possible therapeutic modality for cancer may be developed using the difference of high copper load in tumors versus low copper load in normal cells [101]. This strategy would convert tumor cellular copper into a potent, specific proteasome inhibitor and apoptosis inducer by application of DSF, or a dithiocarbamate, leading to the formation of dithiocarbamate-based coordination copper compounds as potent proteasome inhibitors in human cancer cells. Thus, this approach could pave the way for the development of nontoxic anticancer therapy. Further clinical studies are needed to further prove this concept.
Acknowledgments
This work was partially supported by grants from the National Cancer Institute (1R01CA120009, 3R01CA120009-04S1 and 5R01CA127258-05, to QPD).
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 2.Burger AM, Seth AK. The ubiquitin-mediated protein degradation pathway in cancer: therapeutic implications. Eur J Cancer. 2004;40:2217–2229. doi: 10.1016/j.ejca.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Frezza M, Schmitt S, Dou QP. Targeting the ubiquitin-proteasome pathway: an emerging concept in cancer therapy. Current topics in medicinal chemistry. 2011;11:2888–2905. doi: 10.2174/156802611798281311. [DOI] [PubMed] [Google Scholar]
- 4.Moore BS, Eustaquio AS, McGlinchey RP. Advances in and applications of proteasome inhibitors. Current opinion in chemical biology. 2008;12:434–440. doi: 10.1016/j.cbpa.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen D, Frezza M, Schmitt S, Kanwar J, QPD Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Current cancer drug targets. 2011;11:239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dou QP. Proteasome inhibition in cancer therapy. Nature Reviews Cancer. 2011 http://www.nature.com/nrc/posters/proteasome/index.html.
- 7.Gerards WL, de Jong WW, Boelens W, Bloemendal H. Structure and assembly of the 20S proteasome. Cellular and molecular life sciences: CMLS. 1998;54:253–262. doi: 10.1007/s000180050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groll M, Huber R, Moroder L. The persisting challenge of selective and specific proteasome inhibition. Journal of peptide science: an official publication of the European Peptide Society. 2009;15:58–66. doi: 10.1002/psc.1107. [DOI] [PubMed] [Google Scholar]
- 9.Arrigo AP, Tanaka K, Goldberg AL, Welch WJ. Identity of the 19S ‘prosome’ particle with the large multifunctional protease complex of mammalian cells (the proteasome) Nature. 1988;331:192–194. doi: 10.1038/331192a0. [DOI] [PubMed] [Google Scholar]
- 10.Hoeller D, Dikic I. Targeting the ubiquitin system in cancer therapy. Nature. 2009;458:438–444. doi: 10.1038/nature07960. [DOI] [PubMed] [Google Scholar]
- 11.Rivett AJ. The multicatalytic proteinase of mammalian cells. Archives of biochemistry and biophysics. 1989;268:1–8. doi: 10.1016/0003-9861(89)90558-4. [DOI] [PubMed] [Google Scholar]
- 12.Schenkein DP. Use of proteasome inhibition in the treatment of lung cancer. Clinical lung cancer. 2004;6(Suppl 2):S89–96. doi: 10.3816/clc.2004.s.021. [DOI] [PubMed] [Google Scholar]
- 13.Dutaud D, Aubry L, Henry L, Levieux D, Hendil KB, Kuehn L, Bureau JP, Ouali A. Development and evaluation of a sandwich ELISA for quantification of the 20S proteasome in human plasma. Journal of immunological methods. 2002;260:183–193. doi: 10.1016/s0022-1759(01)00555-5. [DOI] [PubMed] [Google Scholar]
- 14.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiological reviews. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 15.Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat Rev. 2003;29(Suppl 1):3–9. doi: 10.1016/s0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto KM. Ubiquitin-dependent proteolysis: its role in human diseases and the design of therapeutic strategies. Molecular genetics and metabolism. 2002;77:44–56. doi: 10.1016/s1096-7192(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 17.Seth A, Kitching R, Landberg G, Xu J, Zubovits J, Burger AM. Gene expression profiling of ductal carcinomas in situ and invasive breast tumors. Anticancer research. 2003;23:2043–2051. [PubMed] [Google Scholar]
- 18.Bruijnincx PC, Sadler PJ. Controlling Platinum, Ruthenium and Osmium Reactivity for Anticancer Drug Design. Advances in inorganic chemistry. 2009;61:1–62. doi: 10.1016/S0898-8838(09)00201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Z, Sadler P. Metals in Medicine. Angew Chem Int Ed. 1999;38:1512–1531. doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Ang W, Dyson P. Classical and Non-Classical Ruthenium-Based Anticancer Drugs: Towards Targeted Chemotherapy. Eur J Inorg Chem. 2006:4003–4018. [Google Scholar]
- 21.Liu HK, Berners-Price SJ, Wang F, Parkinson JA, Xu J, Bella J, Sadler PJ. Diversity in guanine-selective DNA binding modes for an organometallic ruthenium arene complex. Angew Chem Int Ed Engl. 2006;45:8153–8156. doi: 10.1002/anie.200602873. [DOI] [PubMed] [Google Scholar]
- 22.Habtemariam A, Melchart M, Fernandez R, Parsons S, Oswald ID, Parkin A, Fabbiani FP, Davidson JE, Dawson A, Aird RE, Jodrell DI, Sadler PJ. Structure-activity relationships for cytotoxic ruthenium(II) arene complexes containing N,N-, N,O-, and O,O-chelating ligands. Journal of medicinal chemistry. 2006;49:6858–6868. doi: 10.1021/jm060596m. [DOI] [PubMed] [Google Scholar]
- 23.Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Fang IM, Yang CH, Lin CP, Yang CM, Chen MS. Effects of pyrrolidine dithiocarbamate, an NF-kappaB inhibitor, on cytokine expression and ocular inflammation in experimental autoimmune anterior uveitis. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2005;21:95–106. doi: 10.1089/jop.2005.21.95. [DOI] [PubMed] [Google Scholar]
- 25.Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, Suarez A, Morgan A, Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. Journal of virology. 2005;79:8014–8023. doi: 10.1128/JVI.79.13.8014-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dou QP, Li B. Proteasome inhibitors as potential novel anticancer agents. Drug Resist Updat. 1999;2:215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- 27.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 28.Orlowski RZ, Dees EC. The role of the ubiquitination-proteasome pathway in breast cancer: applying drugs that affect the ubiquitin-proteasome pathway to the therapy of breast cancer. Breast Cancer Res. 2003;5:1–7. doi: 10.1186/bcr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell death and differentiation. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 30.Milacic V, Chen D, Ronconi L, Landis-Piwowar KR, Fregona D, Dou QP. A novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer research. 2006;66:10478–10486. doi: 10.1158/0008-5472.CAN-06-3017. [DOI] [PubMed] [Google Scholar]
- 31.Milacic V, Chen D, Giovagnini L, Diez A, Fregona D, Dou QP. Pyrrolidine dithiocarbamate-zinc(II) and -copper(II) complexes induce apoptosis in tumor cells by inhibiting the proteasomal activity. Toxicology and applied pharmacology. 2008;231:24–33. doi: 10.1016/j.taap.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodisman J, Hagrman D, Tacka KA, Souid AK. Analysis of cytotoxicities of platinum compounds. Cancer Chemother Pharmacol. 2006;57:257–267. doi: 10.1007/s00280-005-0041-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang X. Fresh platinum complexes with promising antitumor activity. Anticancer Agents Med Chem. 2010;10:396–411. doi: 10.2174/1871520611009050396. [DOI] [PubMed] [Google Scholar]
- 34.Reedijk J. Metal-Ligand Exchange Kinetics in Platinum and Ruthenium Complexes. Platinum Met Rev. 2008;52:2–11. [Google Scholar]
- 35.Kostova I. Platinum complexes as anticancer agents. Recent patents on anti-cancer drug discovery. 2006;1:1–22. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]
- 36.Marzano C, Bettio F, Baccichetti F, Trevisan A, Giovagnini L, Fregona D. Antitumor activity of a new platinum(II) complex with low nephrotoxicity and genotoxicity. Chemico-biological interactions. 2004;148:37–48. doi: 10.1016/j.cbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Ronconi L, Fregona D. The Midas touch in cancer chemotherapy: from platinum- to gold-dithiocarbamato complexes. Dalton Trans. 2009:10670–10680. doi: 10.1039/b913597a. [DOI] [PubMed] [Google Scholar]
- 38.Fricker S. Medical uses of gold compounds: past, present and future. Gold Bulletin. 1996;29:53–60. [Google Scholar]
- 39.Mirabelli CK, Johnson RK, Hill DT, Faucette LF, Girard GR, Kuo GY, Sung CM, Crooke ST. Correlation of the in vitro cytotoxic and in vivo antitumor activities of gold(I) coordination complexes. J Med Chem. 1986;29:218–223. doi: 10.1021/jm00152a009. [DOI] [PubMed] [Google Scholar]
- 40.Milacic V, Fregona D, Dou QP. Gold complexes as prospective metal-based anticancer drugs. Histology and histopathology. 2008;23:101–108. doi: 10.14670/HH-23.101. [DOI] [PubMed] [Google Scholar]
- 41.Fricker SP. A Screening Strategy for Metal Antitumor Agents as Exemplified by Gold(III) Complexes. Metal-based drugs. 1999;6:291–300. doi: 10.1155/MBD.1999.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronconi L, Marzano C, Zanello P, Corsini M, Miolo G, Macca C, Trevisan A, Fregona D. Gold(III) dithiocarbamate derivatives for the treatment of cancer: solution chemistry, DNA binding, and hemolytic properties. Journal of medicinal chemistry. 2006;49:1648–1657. doi: 10.1021/jm0509288. [DOI] [PubMed] [Google Scholar]
- 43.Messori L, Marcon G, Orioli P. Gold(III) compounds as new family of anticancer drugs. Bioinorganic chemistry and applications. 2003:177–187. doi: 10.1155/S1565363303000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronconi L, Giovagnini L, Marzano C, Bettio F, Graziani R, Pilloni G, Fregona D. Gold dithiocarbamate derivatives as potential antineoplastic agents: design, spectroscopic properties, and in vitro antitumor activity. Inorganic chemistry. 2005;44:1867–1881. doi: 10.1021/ic048260v. [DOI] [PubMed] [Google Scholar]
- 45.Bodenner DL, Dedon PC, Keng PC, Borch RF. Effect of diethyldithiocarbamate on cis-diamminedichloroplatinum(II)-induced cytotoxicity, DNA cross-linking, and gamma-glutamyl transpeptidase inhibition. Cancer research. 1986;46:2745–2750. [PubMed] [Google Scholar]
- 46.Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB. Solution structure of a cisplatin-induced DNA interstrand cross-link. Science. 1995;270:1842–1845. doi: 10.1126/science.270.5243.1842. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Frezza M, Milacic V, Ronconi L, Fan Y, Bi C, Fregona D, Dou QP. Inhibition of tumor proteasome activity by gold-dithiocarbamato complexes via both redox-dependent and -independent processes. Journal of cellular biochemistry. 2010;109:162–172. doi: 10.1002/jcb.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geraki K, Farquharson MJ, Bradley DA. Concentrations of Fe, Cu and Zn in breast tissue: a synchrotron XRF study. Physics in medicine and biology. 2002;47:2327–2339. doi: 10.1088/0031-9155/47/13/310. [DOI] [PubMed] [Google Scholar]
- 49.McAuslan BR, Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Experimental cell research. 1980;130:147–157. doi: 10.1016/0014-4827(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 50.Frangoulis M, Georgiou P, Chrisostomidis C, Perrea D, Dontas I, Kavantzas N, Kostakis A, Papadopoulos O. Rat epigastric flap survival and VEGF expression after local copper application. Plastic and reconstructive surgery. 2007;119:837–843. doi: 10.1097/01.prs.0000252000.59231.5e. [DOI] [PubMed] [Google Scholar]
- 51.Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK, Roy S. Copper-induced vascular endothelial growth factor expression and wound healing. American journal of physiology Heart and circulatory physiology. 2002;282:H1821–1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 52.Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, De Carvalho M, Mesri EA, Robins DM, Dick RD, Brewer GJ, Merajver SD. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer research. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 53.Yoshii J, Yoshiji H, Kuriyama S, Ikenaka Y, Noguchi R, Okuda H, Tsujinoue H, Nakatani T, Kishida H, Nakae D, Gomez DE, De Lorenzo MS, Tejera AM, Fukui H. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. International journal of cancer Journal international du cancer. 2001;94:768–773. doi: 10.1002/ijc.1537. [DOI] [PubMed] [Google Scholar]
- 54.Chakravarty PK, Ghosh A, Chowdhury JR. Zinc in human malignancies. Neoplasma. 1986;33:85–90. [PubMed] [Google Scholar]
- 55.Margalioth EJ, Schenker JG, Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz AE, Leddicotte GW, Fink RW, Friedman EW. Trace elements in noraml and malignant human breast tissue. Surgery. 1974;76:325–329. [PubMed] [Google Scholar]
- 57.Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Molecular cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manning DL, Robertson JF, Ellis IO, Elston CW, McClelland RA, Gee JM, Jones RJ, Green CD, Cannon P, Blamey RW, et al. Oestrogen-regulated genes in breast cancer: association of pLIV1 with lymph node involvement. Eur J Cancer. 1994;30A:675–678. doi: 10.1016/0959-8049(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 61.Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Provinciali M, Di Stefano G, Fabris N. Dose-dependent opposite effect of zinc on apoptosis in mouse thymocytes. International journal of immunopharmacology. 1995;17:735–744. doi: 10.1016/0192-0561(95)00063-8. [DOI] [PubMed] [Google Scholar]
- 63.Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer science. 2008;99:1515–1522. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Federico A, Iodice P, Federico P, Del Rio A, Mellone MC, Catalano G. Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. European journal of clinical nutrition. 2001;55:293–297. doi: 10.1038/sj.ejcn.1601157. [DOI] [PubMed] [Google Scholar]
- 65.Prasad AS, Beck FW, Doerr TD, Shamsa FH, Penny HS, Marks SC, Kaplan J, Kucuk O, Mathog RH. Nutritional and zinc status of head and neck cancer patients: an interpretive review. Journal of the American College of Nutrition. 1998;17:409–418. doi: 10.1080/07315724.1998.10718787. [DOI] [PubMed] [Google Scholar]
- 66.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Archives of biochemistry and biophysics. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cvek B, Milacic V, Taraba J, Dou QP. Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. Journal of medicinal chemistry. 2008;51:6256–6258. doi: 10.1021/jm8007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta psychiatrica Scandinavica Supplementum. 1992;369:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 69.Vallari RC, Pietruszko R. Human aldehyde dehydrogenase: mechanism of inhibition of disulfiram. Science. 1982;216:637–639. doi: 10.1126/science.7071604. [DOI] [PubMed] [Google Scholar]
- 70.Meyer RE. Prospects for a rational pharmacotherapy of alcoholism. The Journal of clinical psychiatry. 1989;50:403–412. [PubMed] [Google Scholar]
- 71.Cen D, Brayton D, Shahandeh B, Meyskens FL, Jr, Farmer PJ. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. Journal of medicinal chemistry. 2004;47:6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 72.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer research. 2006;66:10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 73.Brar SS, Grigg C, Wilson KS, Holder WD, Jr, Dreau D, Austin C, Foster M, Ghio AJ, Whorton AR, Stowell GW, Whittall LB, Whittle RR, White DP, Kennedy TP. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Molecular cancer therapeutics. 2004;3:1049–1060. [PubMed] [Google Scholar]
- 74.Cen D, Gonzalez RI, Buckmeier JA, Kahlon RS, Tohidian NB, Meyskens FL., Jr Disulfiram induces apoptosis in human melanoma cells: a redox-related process. Molecular cancer therapeutics. 2002;1:197–204. [PubMed] [Google Scholar]
- 75.Burkitt MJ, Bishop HS, Milne L, Tsang SY, Provan GJ, Nobel CS, Orrenius S, Slater AF. Dithiocarbamate toxicity toward thymocytes involves their copper-catalyzed conversion to thiuram disulfides, which oxidize glutathione in a redox cycle without the release of reactive oxygen species. Archives of biochemistry and biophysics. 1998;353:73–84. doi: 10.1006/abbi.1998.0618. [DOI] [PubMed] [Google Scholar]
- 76.Chen SH, Liu SH, Liang YC, Lin JK, Lin-Shiau SY. Death signaling pathway induced by pyrrolidine dithiocarbamate-Cu(2+) complex in the cultured rat cortical astrocytes. Glia. 2000;31:249–261. doi: 10.1002/1098-1136(200009)31:3<249::aid-glia60>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 77.Chen D, Peng F, Cui QC, Daniel KG, Orlu S, Liu J, Dou QP. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Frontiers in bioscience: a journal and virtual library. 2005;10:2932–2939. doi: 10.2741/1749. [DOI] [PubMed] [Google Scholar]
- 78.Chen J, Du C, Kang J, Wang J. Cu2+ is required for pyrrolidine dithiocarbamate to inhibit histone acetylation and induce human leukemia cell apoptosis. Chemico-biological interactions. 2008;171:26–36. doi: 10.1016/j.cbi.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Marikovsky M, Nevo N, Vadai E, Harris-Cerruti C. Cu/Zn superoxide dismutase plays a role in angiogenesis. International journal of cancer Journal international du cancer. 2002;97:34–41. doi: 10.1002/ijc.1565. [DOI] [PubMed] [Google Scholar]
- 80.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. British journal of cancer. 2011;104:1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans RG, Engel C, Wheatley C, Nielsen J. Modification of the sensitivity and repair of potentially lethal damage by diethyldithiocarbamate during and following exposure of plateau-phase cultures of mammalian cells to radiation and cis-diamminedichloroplatinum(II) Cancer research. 1982;42:3074–3078. [PubMed] [Google Scholar]
- 83.Hacker MP, Ershler WB, Newman RA, Gamelli RL. Effect of disulfiram (tetraethylthiuram disulfide) amd diethyldithiocarbamate on the bladder toxicity and antitumor activity of cyclophosphamide in mice. Cancer research. 1982;42:4490–4494. [PubMed] [Google Scholar]
- 84.Bodenner DL, Dedon PC, Keng PC, Katz JC, Borch RF. Selective protection against cis-diamminedichloroplatinum(II)-induced toxicity in kidney, gut, and bone marrow by diethyldithiocarbamate. Cancer research. 1986;46:2751–2755. [PubMed] [Google Scholar]
- 85.Lin J, Haffner MC, Zhang Y, Lee BH, Brennen WN, Britton J, Kachhap SK, Shim JS, Liu JO, Nelson WG, Yegnasubramanian S, Carducci MA. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. The Prostate. 2011;71:333–343. doi: 10.1002/pros.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brahemi G, Kona FR, Fiasella A, Buac D, Soukupova J, Brancale A, Burger AM, Westwell AD. Exploring the structural requirements for inhibition of the ubiquitin E3 ligase breast cancer associated protein 2 (BCA2) as a treatment for breast cancer. Journal of medicinal chemistry. 2010;53:2757–2765. doi: 10.1021/jm901757t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lovborg H, Oberg F, Rickardson L, Gullbo J, Nygren P, Larsson R. Inhibition of proteasome activity, nuclear factor-KappaB translocation and cell survival by the antialcoholism drug disulfiram. International journal of cancer Journal international du cancer. 2006;118:1577–1580. doi: 10.1002/ijc.21534. [DOI] [PubMed] [Google Scholar]
- 88.Wickstrom M, Danielsson K, Rickardson L, Gullbo J, Nygren P, Isaksson A, Larsson R, Lovborg H. Pharmacological profiling of disulfiram using human tumor cell lines and human tumor cells from patients. Biochemical pharmacology. 2007;73:25–33. doi: 10.1016/j.bcp.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 89.Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug safety: an international journal of medical toxicology and drug experience. 1999;20:427–435. doi: 10.2165/00002018-199920050-00003. [DOI] [PubMed] [Google Scholar]
- 90.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. The Journal of experimental medicine. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu GH, Wang SR, Wang B, Kong BH. Inhibition of nuclear factor-kappaB by an antioxidant enhances paclitaxel sensitivity in ovarian carcinoma cell line. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2006;16:1777–1782. doi: 10.1111/j.1525-1438.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 92.Chinery R, Beauchamp RD, Shyr Y, Kirkland SC, Coffey RJ, Morrow JD. Antioxidants reduce cyclooxygenase-2 expression, prostaglandin production, and proliferation in colorectal cancer cells. Cancer research. 1998;58:2323–2327. [PubMed] [Google Scholar]
- 93.Arima N, Arimura K, Tokito Y, Sakaki Y, Matsushita K, Orihara K, Akimoto M, Ozaki A, Kukita T, Hagiwara T, Hamada H, Tei C. HTLV-I Tax protein inhibits apoptosis induction but not G1 arrest by pyrrolidinedithiocarbamate, an anti-oxidant, in adult T cell leukemia cells. Experimental hematology. 2004;32:195–201. doi: 10.1016/j.exphem.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 94.Li Q, Yu YY, Zhu ZG, Ji YB, Zhang Y, Liu BY, Chen XH, Lin YZ. Effect of NF-kappaB constitutive activation on proliferation and apoptosis of gastric cancer cell lines. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. 2005;37:105–110. doi: 10.1159/000084541. [DOI] [PubMed] [Google Scholar]
- 95.Morais C, Pat B, Gobe G, Johnson DW, Healy H. Pyrrolidine dithiocarbamate exerts anti-proliferative and pro-apoptotic effects in renal cell carcinoma cell lines. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:3377–3388. doi: 10.1093/ndt/gfl543. [DOI] [PubMed] [Google Scholar]
- 96.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005;7:R897–908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, James ND, Spooner D, Poole CJ, Rea DW, Palmer DH. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. British journal of cancer. 2007;96:104–109. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carta F, Aggarwal M, Maresca A, Scozzafava A, McKenna R, Masini E, Supuran CT. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. Journal of medicinal chemistry. 2012 doi: 10.1021/jm300031j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burger AM, Gao Y, Amemiya Y, Kahn HJ, Kitching R, Yang Y, Sun P, Narod SA, Hanna WM, Seth AK. A novel RING-type ubiquitin ligase breast cancer-associated gene 2 correlates with outcome in invasive breast cancer. Cancer research. 2005;65:10401–10412. doi: 10.1158/0008-5472.CAN-05-2103. [DOI] [PubMed] [Google Scholar]
- 100.Gu JW, Young E, Busby B, Covington J, Tan W, Johnson JW. Oral administration of pyrrolidine dithiocarbamate (PDTC) inhibits VEGF expression, tumor angiogenesis and growth of breast cancer in female mice. Cancer biology & therapy. 2009;8 doi: 10.4161/cbt.8.6.7689. [DOI] [PubMed] [Google Scholar]
- 101.Daniel KG, Chen D, Yan B, Dou QP. Copper-binding compounds as proteasome inhibitors and apoptosis inducers in human cancer. Frontiers in bioscience: a journal and virtual library. 2007;12:135–144. doi: 10.2741/2054. [DOI] [PubMed] [Google Scholar]